Review
Clinical Considerations in the Approach to Vancomycin-Resistant Enterococci: A Narrative
Review
John W. Beale1, Marina Durward-Diioia2
doi: http://dx.doi.org/10.5195/ijms.2022.1010
Volume 10, Number 2: 202-209
Received 16 04 2021:
Rev-request 18 05 2021:
Rev-request 03 08 2021:
Rev-request 03 11 2021:
Rev-request 01 02 2022:
Rev-recd 19 05 2021:
Rev-recd 25 08 2021:
Rev-recd 22 11 2021:
Rev-recd 28 03 2022:
Accepted 04 04 2022
ABSTRACT
Vancomycin-Resistant Enterococci (VRE) increasingly colonize and infect assorted patient populations throughout the
world, maintaining a continual reservoir of opportunistic pathogens with varying antibiotic
resistance. Here we present the current general epidemiology and classification of
these pathogens within the scope of healthcare-associated infections (HAIs). Risk
factors for colonization and conditions for subsequent infection are reviewed, along
with infection characteristics. Current infection control protocols and their effectiveness,
selected evidence-based medical therapies, and ongoing research into alternative therapies
are summarized.
Keywords:
Vancomycin-resistant enterococci;
Enterococcus faecalis;
Enterococcus faecium;
Vancomycin resistance;
Healthcare-associated infection;
Nosocomial infection (Source: MeSH-NLM).
Introduction
First identified in the United Kingdom and France during the 1980s, Enterococci possessing vancomycin-resistance (VRE) colonizes patients in the United States (U.S.)
at increasing rates.1,2 Infections stemming from VRE colonization account for approximately 30% of all healthcare-associated
Enterococci infections in the U.S.3 During the late 2000s, VRE-related hospitalizations doubled in the U.S. alone.1 Worldwide reported VRE surveillance data varies widely by continent and country.
Reports from Africa are diverse, with the published prevalence of VRE among human
isolates varying from 2.5% to 44.3%.4 2016 data from the European Antimicrobial Resistance Surveillance Network (EARS-Net)
reported that between 25% and 50% of surveillance isolates of E. faecium from Ireland, Eastern, and Southern Europe were positive for VRE.5 While a U.S. Centers for Disease Control (CDC) report in 2019 showed decreasing cases
over the last several years from 84,800 confirmed VRE infections in hospitalized patients
in 2012 to 54,500 cases in 2017; the prevalence of vancomycin resistance is still
alarmingly high at 30% of all healthcare-associated infections.6 Variable surveillance data from Asia and Australasia suggest a low prevalence of
VRE compared to Europe and the U.S. for instance, a 5-year study in Singapore found
a prevalence of vancomycin resistance in isolates at 0.4-0.7%, but these rates appear
to be increasing.7 The variable yet increasing prevalence of vancomycin resistance should concern physicians,
scientists, and patients worldwide.
Colonization rate increases may be attributed to Enterococci's natural habitat and genetic structure. One of many bacterial species composing
normal human enteric microbiota, Enterococci gaining vancomycin-resistance are perfectly positioned for enhanced opportunistic
pathogenicity. Enterococci already possess intrinsic resistance to many antibacterial agents, including β-lactams
and aminoglycosides.8–10 Existence with other commensal bacteria provides ample opportunities for acquiring
vancomycin resistance via transposition of resistance-containing plasmids.11–13 Nine different phenotypes – VanA, VanB, VanC, VanD, VanE, VanG, VanL, VanM, and VanN
– named for the vancomycin-resistance gene (van) expressed currently describe degrees of vancomycin-resistance and pathogenicity
within Enterococci.11,14,15 For example, E. faecium most frequently expresses the vanA gene and thus is most frequently associated with the VanA phenotype, which identifies the highest vancomycin resistance and, consequently,
the highest pathogenicity.10,14 The VanB phenotype identifies expression of the vanB gene and an intermediate level of vancomycin-resistance that, while less pathogenic,
still commonly appears in surveillance cultures of patient populations.10,15–17 VanC phenotype Enterococci express the vanC gene and possess much lower vancomycin-resistance.10,15 VRE are thus a family of variably-resistant opportunistic pathogens, with E. faecium and E. faecalis being the most commonly identified.8–10,18 Increasing VRE prevalence intensifies the need to quickly identify patients at risk
for colonization and infection to treat colonized and infected patients with the potential
to lower overall colonization rates.
This review aims to present the general epidemiology and medical management of healthcare-associated
VRE infections. In order to clarify the variable at-risk patient populations, we reviewed
essential factors for colonization. We recently reported conditions for subsequent
infection, followed by a review of infection control protocols, which are of heightened
importance in health care settings. Further, recent updates to the pharmacological
interventions and alternative therapies, including rebiosis, are discussed and compared.
Methods
A narrative review of English language literature from 1994 to August 2019 was utilized
to assess the historical development of vancomycin resistance within the Enterococcus family. This timeline was revisited prior to publication and updated to include the
time frame to March 2022. Scale for Assessment of Narrative Review Articles (SANRA)
was used to guide appropriate research methods.19 The primary research method was an online search conducted in September 2019 on Google
Scholar and PubMed. Search terms included: vancomycin resistance OR vre OR “vancomycin-resistant”
OR multidrug-resistant OR mdro OR infec* AND enterococc* OR “E. faecalis” OR “E. faecium”
OR “enterococcus faecalis” OR “enterococcus faecium” OR microbiome OR microbiota.
Meta-analyses and systematic reviews were given a narrower time frame, namely the
past 10 years compared to case reports or series and other literature reviews or position
papers. This allowed for more recent data on current treatment practices and protocols
while allowing a broader scope for assessing the historical development and response
to vancomycin-resistant Enterococci. The competencies of evidence-based medicine were utilized when developing inclusion/exclusion
criteria.20 These competencies include recognizing a problem, retrieving and critically appraising
the literature, and intergrating information found. Papers dealing specifically with
human models were preferred; however, some animal model studies were included due
to a lack of data with human models.
Inclusion required:
- Title or abstract inclusion of at least two of the search term(s) OR
- Significant (two+ pages) discussion of at least two of the search terms within the
body of the paper OR
- Position papers whose content would apply to at least two of the search terms, even
if not explicitly stated.
Exclusion required:
- Any paper published more than 25 years ago at the time of search (1994 or earlier)
- Any meta-analysis or systematic review published more than 10 years ago at the time
of search (2009 or earlier)
- Any paper that contained only one search term and failed to meet the inclusion criteria
outlined above
- Any paper that included one or more search terms whose primary focus was either another
form of drug resistance or another species of bacteria (e.g., methicillin-resistant
Staphylococcus aureus).
Results and Discussion
Colonization and Infections
Colonization, or the incorporation of a microorganism into a host, occurs through
the interaction of the host with a reservoir of that microorganism. Studies by Hamel
et al. in 2010 and Kaki et al. in 2014 identified VRE colonized patients and contaminated surfaces within hospitals
or care centers as possible VRE reservoirs.21,22 Enterococci inhabit every human colon, but colonization with VRE rarely occurs among healthy
populations.23 Further, in a recent large study (n=674 including controls) of healthcare personnel
and their rates of colonization with multi-drug resistant organisms (MDROs), Decker
et al. found that there were not any healthcare workers or control subjects positive for
VRE colonization, including those in contact with MDRO+ patients.24 A meta-analysis of 37 studies found that 10% of patients in Intensive Care Units
(ICU) are already colonized with VRE at admission, and an additional 10% were colonized
during their ICU stay.23,25 A meta-analysis of dialysis patients, who are typically immunocompromised in the
U.S. found that more than 6% are colonized with VRE.26
VRE colonization risk is multifactorial. Recent high-dose antibiotic use, especially
vancomycin, is the most frequently identified risk factor in multiple studies.13,22,26 Surgical, oncological, and dialysis patients demonstrate increased risk, especially
when recovery requires ICU services.3,10,23,25–28 Patients sharing a room with a VRE colonized patient have a one in three chance of
becoming colonized during hospitalization.21,22,29 Acquired immunodeficiency from Human Immunodeficiency Virus (HIV) infection or medically-induced
immunosuppression may also increase VRE colonization risk.18,27,28,30–32
Immunocompromised patients requiring recurrent medical interventions within a hospital
or long-term care center thus comprise both the highest risk group for colonization
and the potentially largest VRE reservoir.3,33 Patients in these circumstances are prime for a VRE-mediated infection when a critical
lapse in immune function occurs. For example, Brennen et al. found only 1% of colonized patients in a nursing facility develop VRE infections.34 Yet Zaas et al. reported that 13% of colonized oncology patients develop VRE infections.35 In a 2008 study, Zirakzadeh et al. found that hematopoietic stem cell transplant (HSCT) patients colonized with VRE
have a significantly higher 100-day mortality rate (45%) compared to non-colonized
patient controls (25%) and are more prone to develop VRE bacteremia (27%) than non-colonized
patients (0%).30 Colonized dialysis patients demonstrate significantly higher VRE infection risk than
to non-colonized patients, especially when recently hospitalized.26 A 2013 meta-analysis by Ziakas et al. found that among ICU patients, VRE infection rates among those colonized can be
anywhere from 0%-45%, yet the infection rate for non-colonized patients consistently
stayed below 2%.23 As recently as 2018, Freedberg et al. found that VRE colonization was associated with a 19% increased risk for death (P<.01)
and a 22% increased risk of infection (P<.01).25 Infection rate discrepancies indicate a predisposition among VRE-colonized patients
to acquire a VRE infection after a major medical procedure.
VRE infections generally correlate with either the location or method of medical intervention
(Figure 1). VRE infections may localize around surgical incisions with limited spread to adjacent
tissues.27 VRE meningitis, while rare, may complicate cranial surgical procedures in colonized
patients.9,18,36 VRE urinary tract infections (UTIs) commonly afflict colonized patients with indwelling
catheters.27 Peritoneal dialysis in patients colonized with VRE may result in VRE peritonitis.9,26,37 Up to 10% of patients undergoing HSCT or solid organ transplants that develop VRE
bacteremia may experience VRE infective endocarditis.9,18,31 These patients may also be more likely to progress to septic shock.30,32
Figure 1
Common Locations of Medical Procedures with Resulting VRE-Mediated Infections.
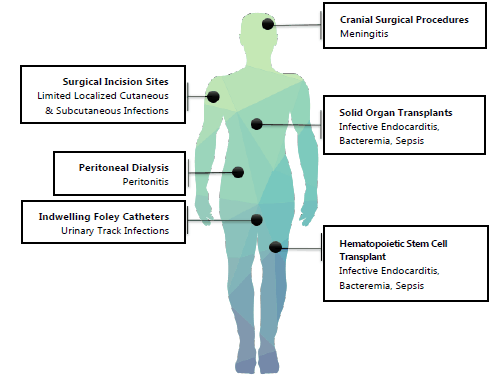
Legend: Note the localized nature of the resulting infections, with two significant exceptions:
Solid organ transplant and hematopoietic stem cell transplant (HSCT). Patients undergoing
these major medical interventions are more likely to suffer from systemic VRE-mediated
infections such as: bacteremia, infective endocarditis (IE), sepsis and possible progression
and worsening to septic shock. This may be due to the highly vascular nature of both
solid organs and bone marrow which facilitate the systemic spread of VRE in susceptible
patients.
All VRE infections cause significant increases in morbidity and mortality when compared
to similar infections with vancomycin-sensitive Enterococci (VSE).10,25,26,30 Mortality rates for surgical patients with VRE bacteremia may be as high as 67%,
nearly double the rate for matched control patients.30,33 VRE infections among leukemia patients may result in mortality rates as high as 73%.18 Mortality rates among VRE-infected allogeneic HSCT recipients with VRE infections
vary between 45% and 80%, depending on the infection.30
Infection Control Protocols
Alarmingly high mortality rates underscore the extensive research and discussion surrounding
VRE infection control protocols. The CDC published recommendations for identifying
and preventing VRE colonization in the mid-1990s.27,28 Recommendations included: active patient surveillance using perianal swabs, culture
on selective media, using gloves and gowns for universal contact precautions (CP),
and isolating VRE-colonized patients during treatment.27,28 These recommendations became the standard in hospital-based VRE infection control
protocols, and for other multidrug-resistant organisms (MDROs). Numerous studies since
the CDC's guidelines were published have evaluated the effectiveness and limitations
of these infection control measures, as discussed below.
Active surveillance of high-risk patients, typically those hospitalized in the intensive
care unit or receiving intravenous antibiotic therapy, has been a mainstay of infection
control; however, limitations primarily involve the time required to culture the surveillance
swabs. Cultures take 48 to 72 hours to grow, during which time yet undetected VRE
may colonize additional patients.17,38 In 2017, Holzknecht et al. demonstrated that Polymerase Chain Reaction (PCR) assay for the vanA and/or vanB genes might significantly reduce the time required to identify VRE-colonized patients
(eight hours for PCR assay compared to 48-72 hours for culture). Very recent PCR assay
development has led to a vastly reduced time frame of two hours to identify VRE, though
costs and availability issues remain.39 This is built on the work of Paule et al., which showed in 2003 that PCR of the vanA gene demonstrates a high specificity (99.7%) and sensitivity (87.1%) for identifying
VRE (compared to about 60% sensitivity for swab and culture).16,17,38,40 Decreased detection time may lead to the earlier implementation of universal CP and
isolation, thus preventing further VRE exposure in unprotected patients and healthcare
workers.
Studies evaluating universal CP in VRE infection control protocols contain positive
but non-specific findings. Research by Calfee et al. in 2003 confirmed the work done earlier by Montecalvo et al. in 1999, reporting a 50% decrease in the incidence of VRE colonization following
CP implementation.27,38,41 Research by Slaughter et al. in 1996 affirmed the use of universal CP; however, they could find no additional
reduction in VRE colonization when using gloves and gowns compared to gloves alone.42 More recent studies by Harris et al. in 2013 and Morgan et al. in 2015 argue for the continued use of universal CP for MDROs, including VRE, while
acknowledging that the clinical research supporting such practice is still lacking.43–45 Recent research by Eichel et al. found that CP did not alter the transmission rates of VRE nor the rate of VRE bacteremia
while hand and environment hygiene were maintained.46
VRE patient isolation protocols focus on maintaining the standard of care. Montecalvo
et al. and Calfee et al. both reported isolation as a component of successful VRE colonization reduction;
however, the degree of benefit that isolation alone provided remains unquantified.27,38,41 Unlike gloves or surveillance cultures, which cause little to no harm to patients,
isolation protocols may actually cause harm to patients. In 2003, Stelfox et al. reported that isolated patients experience two adverse events during treatment compared
to one for non-isolated patients.47 The charts of isolated patients contained fewer vital sign records, fewer physician
progress notes, and elevated complaint and dissatisfaction levels at discharge.47 While evidence supports isolation as a component of VRE infection control protocols;
concerted efforts must ensure these patients receive the same standard of care during
treatment compared to their non-isolated counterparts.
The CDC continually updates practice guidelines for VRE and other MDROs, advocating
for the effective use of infection control measures in a multi-disciplinary approach
that emphasizes prevention as well as treatment.48 Prevention methods include sterilizing medical equipment, using anti-bacterial washes
on patients, and hand hygiene.48 In 2019, Messler et al. reported that octenidine-based body washing reduced VRE colonization by 65% in a
German surgical ICU population.40 This infection control technique, alongside established recommendations, may combat
rising VRE colonization rates more effectively.
Medical Management
Despite the best efforts of healthcare teams and continual refinement of infection
control protocols, VRE infections continually plague susceptible patients. Proper
culture and resistance profiling of patient isolates are essential to ensure patients
receive the most appropriate course of treatment. Few effective antibacterial agents
remain to treat vancomycin-resistant enterococcal infections. Table 1 summarizes commonly cited medical therapies that are now or have been indicated for
VRE infections, including their class and mechanism of action. Currently, the only
antibiotic approved by the U.S. Food and Drug Administration (FDA) for medical management
of VRE-mediated infections is linezolid, an oxazolidinone. While only bacteriostatic
to VRE, linezolid has been successfully used as a monotherapy in several VRE infective
endocarditis cases.13,31 VRE-mediated UTIs and central nervous system infections also respond well to linezolid
monotherapy.13,36 Daptomycin, a cyclic lipopeptide, has a bactericidal action against VRE in certain
disease states and may be used for both VRE-mediated UTIs and infective endocarditis.8,13,32 A recent comparison study revealed that linezolid was associated with a significantly
lower rate of clinical failure compared to the standard dose of daptomycin.49 The same study found that higher doses of daptomycin may overcome some of the clinical
failures.
Table 1.
Medical Therapies Indicated for VRE infections.
| Medication |
Class |
Mechanism |
Reported VRE Efficacy |
Monotherapy or Combination |
| Linezolid |
Oxazolidinone |
Protein synthesis inhibitor – Binds the 23S subunit of ribosomal 50S unit |
*IE, UTI, Meningitis, Peritonitis, Bacteremia
|
Monotherapy |
| Daptomycin |
Lipopeptide (cyclic) |
Cell membrane depolarizer – Inhibits membrane functionality, decreasing DNA, RNA,
and protein synthesis
|
IE, UTI |
Monotherapy or in combination with Ceftaroline |
| Tedizolid |
Oxazolidinone |
Protein synthesis inhibitor – Binds the ribosomal 50S unit |
Bacteremia, IE |
Monotherapy |
| Tigecycline |
Glycylcycline |
Protein synthesis inhibitor – Binds the ribosomal 30S unit |
UTI, Meningitis |
Monotherapy |
| Quinupristin/Dalfopristin |
Streptogramin |
Protein synthesis inhibitor – Binds the ribosomal 50S unit |
Bacteremia, Meningitis, IE |
Combination |
| Chloramphenicol |
Amphenicol |
Protein synthesis inhibitor – Binds the ribosomal 50S unit |
Bacteremia, Meningitis |
Monotherapy |
*IE = infective endocarditis, UTI = urinary tract infection.
A recent study by Kelly et al. found that most patients receiving daptomycin for VRE infections had no side effects
at a dose of 8-12mg/kg/day.50 Further, a cost analysis found that these therapies are similar, with linezolid being
slightly more cost-effective in the U.S.51 Other medications once indicated for VRE infections, such as chloramphenicol and
quinupristin/dalfopristin, have fallen into disuse due to low bacteriostatic/bactericidal
activity or side effects requiring cessation of medical therapy.13,30
Current VRE antimicrobial therapy relies heavily on two primary agents: linezolid
and daptomycin, both of which have a normal incidence of notable adverse events in
patients. Linezolid can lead to the central nervous system and gastrointestinal symptoms
in up to 9.8% of patients, including headache, nausea, vomiting, and diarrhea.52 Daptomycin is reported to be associated with myopathies at higher doses, neuropathy,
and acute eosinophilic pneumonia, though this is considered rare.53,54 Additionally, both linezolid and daptomycin use can lead to anemia, thrombocytopenia,
and renal insufficiency in patients.55 The prevalence of these adverse events underscores the importance of antibiotic development
against VRE.
The World Health Organization (WHO), CDC, and other national and international organizations
continually urge pharmaceutical and academic entities to develop novel regimens.6,56 VRE resistance to linezolid, though currently a rare occurrence, only accentuates
the need for new approaches to VRE infection management.8,13 A new oxazolidinone, tedizolid, may be efficacious against linezolid-resistant VRE
strains, though the FDA does not currently approve it for that indication.13,32 Recent investigations into the use of oritavancin, a lipoglycopeptide, and omadacycline,
a tetracycline, are showing significant efficacy against VRE in small studies, though
more coordinated clinical studies are required.57–59 In vitro studies exploring combinations of daptomycin and ceftaroline, a fifth-generation
cephalosporin, showed promise against VRE infections; however, Chuang et al. in 2017 found no significant difference in mortality between patients receiving
the combination therapy compared to daptomycin monotherapy.8,13,56
High mortality rates underscore the need for effective antibiotics against VRE.27 Two studies examining VRE bacteremia in transplant patients reported 80% and 100%
mortality rates despite treatment with linezolid, daptomycin, and quinupristin/dalfopristin.13,30 While VRE infection may not have been the sole cause of death in all instances, reported
mortality rates would not have been this high without VRE infection.27,30,32 Further research must focus on finding alternative antimicrobial therapies or combination
therapies that provide more significant efficacy against VRE infections.
Potential Therapies
New research into alternative treatment options is producing promising results. Our
current understanding of the human microbiome and its synergistic effects on health
has led to new, targeted treatment modalities affecting several physiological processes,
including metabolism and the immune response.60,61 Colonic dysbiosis, or the disruption of normal enteric microbiota favoring opportunistic
infections, is a proven component of disease pathogenesis in Clostridioides difficile infections, irritable bowel syndrome, and Crohn's disease.60,61 Currently, research examining the links between colonic dysbiosis and VRE colonization
is underway by multiple groups.60–64 This research may lead to new treatment paradigms that can reduce VRE colonization
rates, morbidity, and mortality associated with VRE infections.
Clinical application of current research offers two different therapeutic approaches:
primary rebiosis and secondary rebiosis. Primary rebiosis consists of integrating
probiotic species, or components of these species, within the human microbiome to
restore normal immune function and prevent seeding by opportunistic pathogens such
as VRE.61–63 Secondary rebiosis consists of integrating a donor microbiome en totum to a dysbiotic individual, most commonly accomplished via Fecal Microbiota Transplant
(FMT).60,64 This procedure isolates and purifies a healthy donor sample for direct implantation
into a dysbiotic colon.60,61
Primary rebiosis shows encouraging results in both animal models and preliminary clinical
trials. A 2018 study by Wasilewska et al. of Streptococcus and Lactobacillus in mouse models confirms earlier reports that probiotic regimens have a two-fold
benefit in combating enteric-related infections: modulating colonic immune responses
to favor healthy gut microbiota and enhancing immune response against opportunistic
pathogens within intestinal lymphoid tissues.65 Research by Li et al. of Lactobacillus extracellular vesicles in worm models suggests that components of this probiotic
species alone may be effective in treating VRE colonization.63 Kim et al. studied Blautia producta in mouse models suggesting that administration in a newly colonized host may restore
natural resistance to VRE colonization after antibiotic administration.66 A 2019 retrospective analysis by Borgmann et al. of probiotic therapy conducted in Ingolstadt, Germany, suggests that adding the
probiotics Saccharomyces boulardii and Escherichia coli Nissle to traditional antibiotic regimens reduces VRE transmission in stroke and
trauma patients without any adverse side effects.62 Following the implementation of probiotic regimens, VRE colonization rates dropped
from 78 patients per year to 51 per year, an overall 35% reduction.62 These studies highlight the potential impact of primary rebiosis as an emerging VRE
therapy that may improve the efficacy of existing antimicrobial regimens.
Secondary rebiosis via FMT may be effective in reducing VRE colonization where other
methods have proven ineffective. First employed in refractory Clostridium difficile infections in 2013, FMT has shown surprising efficacy.60,61 Research utilizing mouse model FMT treatments for VRE colonization reduced overall
VRE load, though the effect was transient.64 In 2018, Davido et al. performed the largest human trial to date utilizing FMT as a treatment to decolonize
VRE, resulting in seven of eight initial study patients remaining VRE free 3 months
post-FMT.64 Ongoing trials will assess whether these limited but encouraging results will hold
up in larger clinical studies.67 FMT has been shown to be relatively safe, with the most common side-effects being
mild and self-limiting increases in flatulence, changes in bowel regularity, and abdominal
bloating and tenderness.68 Identification and screening of healthy donor material play a large role in mitigating
the risks associated with the procedure.68 Directly replacing a patient's colonized colonic microbiome with a healthy, VRE-free
microbiome may provide the means to greatly reduce the functional reservoir of VRE
and prevent continued colonization.
Conclusion
Since physicians have identified vancomycin-resistant Enterococci, our understanding of this family of multidrug-resistant, opportunistic pathogens
has grown exponentially. While this body of evidence has grown, we are still looking
for the most appropriate measures to limit the spread of antibiotic-resistant infections.
Clinical cases and meta-analyses have provided clues into the reservoirs of VRE and
the patient populations most at risk from VRE colonization. Incredibly high morbidity
and mortality rates have prompted the development of VRE infection control protocols
that have been implemented, studied, and critiqued for their relative effectiveness.
Further, the development of cost-effective rapid diagnostic testing may limit the
spread of unidentified VRE infections in healthcare settings. Current medical therapies
for VRE infections are unfortunately limited and resistance to linezolid has been
reported but is not widespread as of yet, adding credence to the cries of the WHO,
CDC, and others for new antimicrobial therapies. Ongoing research into the human microbiome
has provided two potentially promising alternative therapy choices, primary and secondary
rebiosis. Though both are still in development, the potential benefits of replacing
a defective microbiome with a healthy and balanced population of normal non-pathogenic
microbes highlight how increased understanding of our own being may provide the key
to discovering how to control and contain vancomycin-resistant Enterococci without the risk of additional antimicrobial resistance. The evidence we have reviewed
here suggests the necessity of a multifactorial approach to VRE: combining surveillance
of at-risk populations, infection control measures, rapid diagnostics, and safe therapies
Conflict of Interest Statement & Funding
The Authors declare that there is no conflict of interest. This work was supported
by start-up grants from ICOM and WVSOM to M.D.
Author Contributions
Conceptualization: JWB, MDD; Data Curation: JWB; Formal Analysis: JWB; Funding Acquisition:
MDD; Investigation: JWB; Methodology: JWB, MDD; Project Administration: MDD; Resources:
JWB, MDD; Software: JWB, MDD; Supervision: MDD; Validation: MDD; Visualization: JWB;
Writing – Original Draft Preparation: JWB, MDD; Writing – Review & Editing: JWB, MDD.
Acknowledgments
None.
References
1. Ramsey AM, Zilberberg MD. Secular Trends of Hospitalization with Vancomycin-Resistant Enterococcus Infection
in the United States, 2000–2006. Infect Control Hosp Epidemiol. 2009;30(2):184–186.
2. Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet Lond Engl. 1988;1(8575-6):57–58.
3. Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, et al. Vital Signs: Preventing Antibiotic-Resistant Infections in Hospitals — United States,
2014. 2016;65(9):7.
4. Osei Sekyere J, Mensah E. Molecular epidemiology and mechanisms of antibiotic resistance in Enterococcus spp.,
Staphylococcus spp., and Streptococcus spp. in Africa: a systematic review from a
One Health perspective. Ann N Y Acad Sci. 2020;1465(1):29–58.
5. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2016. Annual Report of the European
Antimicrobial Resistance Surveillance Network (EARS-Net). Eur Cent Dis Control. Published online 2017.
6. Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.); 2019.
7. Molton JS, Tambyah PA, Ang BSP, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective
from Asia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;56(9):1310–1318.
8. Smith JR, Barber KE, Raut A, Aboutaleb M, Sakoulas G, Rybak MJ. B-Lactam combinations with daptomycin provide synergy against vancomycin-resistant
Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother. Published online February 1, 2015:dkv007.
9. Mazuski JE. Vancomycin-Resistant Enterococcus: Risk Factors, Surveillance, Infections, and Treatment. Surg Infect. 2008;9(6):567–571.
10. Wade JJ, Uttley AH. Resistant enterococci–mechanisms, laboratory detection and control in hospitals. J Clin Pathol. 1996;49(9):700–703.
11. Boyce JM, Opal SM, Chow JW, Zervos MJ, Potter-Bynoe G, Sherman CB, et al. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class
vancomycin resistance. J Clin Microbiol. 1994;32(5):1148–1153.
12. Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319(3):157–161.
13. Crank C, O'Driscoll T. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations,
and optimal management. Infect Drug Resist. Published online July 2015:217.
14. Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993;37(8):1563–1571.
15. Kang M, Xie Y, He C, Chen ZX, Guo L, Yang Q, et al. Molecular characteristics of vancomycin-resistant Enterococcus faecium from a tertiary
care hospital in Chengdu, China: Molecular characteristics of VRE in China. Eur J Clin Microbiol Infect Dis. 2014;33(6):933–939.
16. Holzknecht BJ, Hansen DS, Nielsen L, Kailow A, Jarløv JO. Screening for vancomycin-resistant enterococci with Xpert® vanA/vanB: diagnostic accuracy
and impact on infection control decision making. New Microbes New Infect. 2017;16:54–59.
17. Paule SM, Trick WE, Tenover FC, Lankford M, Cunningham S, Stosor V, et al. Comparison of PCR Assay to Culture for Surveillance Detection of Vancomycin-Resistant
Enterococci. J Clin Microbiol. 2003;41(10):4805–4807.
18. Avery R, Kalaycio M, Pohlman B, Sobecks R, Luczkowski E, Andresen S, et al. Early vancomycin-resistant enterococcus (VRE) bacteremia after allogeneic bone marrow
transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant. 2005;35(5):497–499.
19. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5.
20. Manchikanti L. Evidence-based medicine, systematic reviews, and guidelines in interventional pain
management, part I: introduction and general considerations. Pain Physician. 2008;11(2):161–186.
21. Hamel M, Zoutman D, O'Callaghan C. Exposure to hospital roommates as a risk factor for health care–associated infection. Am J Infect Control. 2010;38(3):173–181.
22. Kaki R, Yu Y, O'Neill C, Lee C, Mertz D, Hamilton Health Sciences Infection Prevention and Control Team. Vancomycin-Resistant Enterococcus (VRE) Transmission and Risk Factors in Contacts
of VRE Carriers. Infect Control Hosp Epidemiol. 2014;35(7):876–879.
23. Ziakas PD, Thapa R, Rice LB, Mylonakis E. Trends and Significance of VRE Colonization in the ICU: A Meta-Analysis of Published
Studies. Ratner AJ, ed. PLoS ONE. 2013;8(9):e75658.
24. Decker BK, Lau AF, Dekker JP, Spalding CD, Sinaii N, Conlan S, et al. Healthcare personnel intestinal colonization with multidrug-resistant organisms. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2018;24(1):82.e1–82.e4.
25. Freedberg DE, Zhou MJ, Cohen ME, Annavajhala MK, Khan S, Moscoso DI, et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission
and risk for subsequent death or infection. Intensive Care Med. 2018;44(8):1203–1211.
26. Zacharioudakis IM, Zervou FN, Ziakas PD, Rice LB, Mylonakis E. Vancomycin-Resistant Enterococci Colonization Among Dialysis Patients: A Meta-analysis
of Prevalence, Risk Factors, and Significance. Am J Kidney Dis. 2015;65(1):88–97.
27. Calfee DP, Giannetta ET, Durbin LJ, Germanson TP, Farr BM. Control of Endemic Vancomycin-Resistant Enterococcus among Inpatients at a University
Hospital. Clin Infect Dis. 2003;37(3):326–332.
28. CDC. Recommendations for preventing the spread of Vancomycin resistance: Recommendations
of the Hospital Infection Control Practices Advisory Committee (HICPAC). Recomm Rep. 1995;44(12):1–13.
29. Cheah ALY, Cheng AC, Spelman D, Nation RL, Kong DCM, McBryde ES. Mathematical modelling of vancomycin-resistant enterococci transmission during passive
surveillance and active surveillance with contact isolation highlights the need to
identify and address the source of acquisition. BMC Infect Dis. 2018;18(1):511.
30. Zirakzadeh A, Gastineau DA, Mandrekar JN, Burke JP, Johnston PB, Patel R. Vancomycin-resistant enterococcal colonization appears associated with increased mortality
among allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41(4):385–392.
31. Archuleta S, Murphy B, Keller MJ. Successful treatment of vancomycin-resistant Enterococcus faecium endocarditis with
linezolid in a renal transplant recipient with human immunodeficiency virus infection. Transpl Infect Dis. 2004;6:117–119.
32. Satlin MJ, Walsh TJ. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant
Enterococcus: Three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017;19(6):e12762.
33. Edmond MB, Ober JF, Dawson JD, Weinbaum DL, Wenzel RP. Vancomycin-Resistant Enterococcal Bacteremia: Natural History and Attributable Mortality. Clin Infect Dis. 1996;23(6):1234–1239.
34. Brennen C, Wagener MM, Muder RR. Vancomycin-resistant Enterococcus faecium in a long-term care facility. J Am Geriatr Soc. 1998;46(2):157–160.
35. Zaas AK, Song X, Tucker P, Perl TM. Risk factors for development of vancomycin-resistant enterococcal bloodstream infection
in patients with cancer who are colonized with vancomycin-resistant enterococci. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;35(10):1139–1146.
36. Hachem R, Afif C, Gokaslan Z, Raad I. Successful Treatment of Vancomycin-Resistant Enterococcus Meningitis with Linezolid. Eu Jou CLin Micro & Inf Dis.:4.
37. Song IJ, Seo JW, Kwon YE, Kim YL, Lim TS, Kang EW, et al. Successful Treatment of Vancomycin-Resistant Enterococcus Peritonitis Using Linezolid
Without Catheter Removal in a Peritoneal Dialysis Patient. Perit Dial Int. 2014;34(2):235–239.
38. Diekema D. Use of Active Surveillance Cultures to Control Vancomycin-Resistant Enterococcus. Clin Infect Dis. 2003;37:1399–1400.
39. He YH, Ruan GJ, Hao H, Xue F, Ma YK, Zhu SN, et al. Real-time PCR for the rapid detection of vanA, vanB and vanM genes. J Microbiol Immunol Infect. 2020;53(5):746–750.
40. Messler S, Klare I, Wappler F, Werner G, Ligges U, Sakka SG, et al. Reduction of nosocomial bloodstream infections and nosocomial vancomycin-resistant
Enterococcus faecium on an intensive care unit after introduction of antiseptic octenidine-based
bathing. J Hosp Infect. 2019;101(3):264–271.
41. Montecalvo MA, Jarvis WR, Uman J, Shay DK, Petrullo C, Rodney K, et al. Infection-Control Measures Reduce Transmission of Vancomycin-Resistant Enterococci
in an Endemic Setting. Ann Intern Med. 1999;131(4):269.
42. Slaughter S. A Comparison of the Effect of Universal Use of Gloves and Gowns with That of Glove
Use Alone on Acquisition of Vancomycin-Resistant Enterococci in a Medical Intensive
Care Unit. Ann Intern Med. 1996;125(6):448.
43. Rubin MA, Samore MH, Harris AD. The Importance of Contact Precautions for Endemic Methicillin-Resistant Staphylococcus
aureus and Vancomycin-Resistant Enterococci. JAMA. 2018;319(9):863.
44. Harris AD, Pineles L, Belton B, Johnson JK, Shardell M, Loeb M, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the
ICU: a randomized trial. JAMA. 2013;310(15):1571–1580.
45. Morgan DJ, Murthy R, Munoz-Price LS, Barnden M, Camins BC, Johnston BL, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus
aureus and vancomycin-resista.nt Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163–1172.
46. Eichel VM, Boutin S, Frank U, Weigand MA, Heininger A, Mutters NT, et al. Impact of discontinuing contact precautions and enforcement of basic hygiene measures
on nosocomial vancomycin-resistant Enterococcus faecium transmission. J Hosp Infect. 2022;121:120–127.
47. Stelfox HT. Safety of Patients Isolated for Infection Control. JAMA. 2003;290(14):1899.
48. Siegel JD, Rhinehart E, Cic R, Jackson M. Management of Multidrug-Resistant Organisms In Healthcare Settings, 2006. Published online 2006:75.
49. Narayanan N, Rai R, Vaidya P, Desai A, Bhowmick T, Weinstein MP. Comparison of linezolid and daptomycin for the treatment of vancomycin-resistant enterococcal
bacteremia. Ther Adv Infect Dis. 2019;6:2049936119828964.
50. Kelly J, Tysall L, Dewar S. Daptomycin susceptibility testing and therapeutic use in enterococcal bloodstream
infection (EBSI) in a setting with high rates of vancomycin-resistant Enterococcus
faecium (VREfm). J Antimicrob Chemother. Published online February 15, 2022:dkac042.
51. McComb MN, Collins CD. Comparative cost-effectiveness of alternative empiric antimicrobial treatment options
for suspected enterococcal bacteremia. Pharmacotherapy. 2014;34(6):537–544.
52. Birmingham MC, Rayner CR, Meagher AK, Flavin SM, Batts DH, Schentag JJ. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience
from a compassionate-use program. Clin Infect Dis Off Publ Infect Dis Soc Am. 2003;36(2):159–168.
53. Gallagher JC, Perez ME, Marino EA, LoCastro LG, Abrardo LA, MacDougall C. Daptomycin therapy for vancomycin-resistant enterococcal bacteremia: a retrospective
case series of 30 patients. Pharmacotherapy. 2009;29(7):792–799.
54. Patel JJ, Antony A, Herrera M, Lipchik RJ. Daptomycin-induced acute eosinophilic pneumonia. WMJ Off Publ State Med Soc Wis. 2014;113(5):199–201.
55. Balli EP, Venetis CA, Miyakis S. Systematic review and meta-analysis of linezolid versus daptomycin for treatment of
vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother. 2014;58(2):734–739.
56. Chuang YC, Chen PY, Lin CY, Chen YC, Wang JT, Chang SC. A retrospective clinical comparison of daptomycin vs daptomycin and a beta-lactam
antibiotic for treating vancomycin-resistant Enterococcus faecium bloodstream infections. Sci Rep. 2018;8(1):1632.
57. Karlowsky JA, Steenbergen J, Zhanel GG. Microbiology and Preclinical Review of Omadacycline. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;69(Suppl 1):S6–S15.
58. Yim J, Smith JR, Rybak MJ. Role of Combination Antimicrobial Therapy for Vancomycin-Resistant Enterococcus faecium
Infections: Review of the Current Evidence. Pharmacotherapy. 2017;37(5):579–592.
59. Mercuro NJ, Davis SL, Zervos MJ, Herc ES. Combatting resistant enterococcal infections: a pharmacotherapy review. Expert Opin Pharmacother. 2018;19(9):979–992.
60. Antao EM, Vincze S, Hanke R, Klimmek L, Suchecka K, Lubke-Becker A, et al. Antibiotic resistance, the 3As and the road ahead. Gut Pathog. 2018;10(1):52.
61. Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease: Curr Opin Gastroenterol. 2015;31(1):69–75.
62. Borgmann S, Rieβ B, Siegmund R, Werner G, Klare I. Treatment with Saccharomyces boulardii and Escherichia coli Nissle is safe and associated
with reduced nosocomial transmission of vanB vancomycin-resistant Enterococcus faecium
on an early rehabilitation ward in Germany: a retrospective analysis. Ther Clin Risk Manag. 2019;Volume 15:343–354.
63. Li M, Lee K, Hsu M, Nau G, Mylonakis E, Ramratnam B. Lactobacillus-derived extracellular vesicles enhance host immune responses against
vancomycin-resistant enterococci. BMC Microbiol. 2017;17(1):66.
64. Davido B, Batista R, Fessi H, Michelon H, Escaut L, Lawrence C, et al. Fecal microbiota transplantation to eradicate vancomycin-resistant enterococci colonization
in case of an outbreak. Médecine Mal Infect. 2019;49(3):214–218.
65. Wasilewska E, Zlotkowska D, Wroblewska B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus
ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate
sodium-induced colitis. J Dairy Sci. 2019;102(1):37–53.
66. Kim SG, Becattini S, Moody TU, Shliaha PV, Littmann ER, Seok R, et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature. 2019;572(7771):665–669.
67. Davido B, Batista R, Fessi H, Salomon J, Dinh A. Impact of faecal microbiota transplantation to eradicate vancomycin-resistant enterococci
(VRE) colonization in humans. J Infect. 2017;75(4):376–377.
68. Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–127.
John W. Beale, 1 Fourth-year Medical Student, Idaho College of Osteopathic Medicine, Meridian, Idaho,
United States.
Marina Durward-Diioia, 2 Ph.D., West Virginia School of Osteopathic Medicine, Lewisburg, West Virginia, United
States.
About the Author: John W. Beale is currently a fourth-year medical student at the Idaho College of
Osteopathic Medicine in Meridian, ID, USA, a four-year program.
Correspondence: John W. Beale, Address: 1401 E Central Dr, Meridian, ID, 83642, United States. Email:
mdiioia@osteo.wvsom.edu
Editor: Francisco J. Bonilla-Escobar
Student Editors: Duha Shellah & Leah Komer
Copyeditor: Madeleine J. Cox
Proofreader: Kiera Liblik
Layout Editor: Cesare Mercalli
Process: Peer-reviewed
Cite as: Beale, JW, Durward-Diioia, M. Clinical Considerations in the Approach to Vancomycin-Resistant
Enterococci: A Narrative Review. Int J Med Stud. 2022 Apr-Jun;10(2):202-9.
Copyright © 2022 John W. Beale, Marina Durward-Diioia
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 2, April 2022
