Secondary Electron Image from Control Renal Tissue. Magnification at 4000x.
Tânia Nogueira1, Mariana Semedo2, Elisabete Cunha3
doi: http://dx.doi.org/10.5195/ijms.2015.107
Volume 3, Number 1: 10-14
Received 09 08 2014: Accepted 19 01 2015
ABSTRACT
Background:Metals such as copper and zinc are crucial in several vital functions in the human body; the absence of these metals can cause serious illness. When in excess, however, they can have toxic effects which may be associated with carcinogenesis, as is described in the literature. Thus, it is important to realize that without these essential metals in their due proportion, the human body could not maintain its proper metabolic function.
Methods:The main goal of this paper was to compare qualitatively and semi-quantitatively the amount of both copper and zinc present in the tumor tissue (tissue from patients who had undergone partial or radical nephrectomy) and in the control tissue (which was adjacent to the tumor tissue). This study was carried out using Scanning Electron Microscopy coupled with X-Ray Microanalysis (SEM-XRM).
Results:There is a different concentrations of copper and zinc in the samples of tumor tissue and controls that were studied.
Conclusion:This work complements previously published results about the presence of metals in the human body and their probable influence on carcinogenesis.
Keywords: Copper; Zinc; Renal Cell Carcinoma; Scanning Electron Microscopy; Electron Probe Microanalysis.
The majority of the chemical elements that compose the periodic table are present in the human organism; some metals are even vital to its functioning. Their absence can create deficiencies in metabolic functions, leading to serious illnesses, while their excessive abundance can be toxic. Copper (Cu) and zinc (Zn), along with other heavy metals, are involved in metabolic processes which regulate energy production. However, despite the fact that they are essential, their high levels cause a toxic effect which can ultimately lead to carcinogenesis. Thus, the aim of this work was to compare the chemical content of these metals in tumor tissue and control tissue (adjacent to the tumor).
Copper (Cu) deficiency is a rare condition in human beings since almost all diets have at least some quantity of this metal and it is essential in low doses only (the recommended value is 0.9mg per day).1 Non-occupational exposure is through ingestion but populations can also be exposed by inhalation or skin contact since copper can be found in surface waters due to the increasing utilization of the element in aquaculture to control algae and pathogens.2
Previous studies showed that in individuals who smoke, there is a higher level of plasmatic Cu; similar findings occur in patients with arteriosclerosis and in patients with periphery arterial disease.3–5 Despite all of these considerations, it is in the study of tumors that the Cu dosage has more interest as its concentrations have been proven to be higher in renal clear-cell carcinoma samples, contrarily to what occurs in liver tumors.6 Therefore, this metal was chosen to study since it is essential to consider the problems related to the ingestion, absorption, and transport of Cu in the blood stream, and the resultant possible toxic effects associated with Cu.
The primary toxic effects of Cu manifest in the liver as this is the organ where Cu accumulates after entering circulation. However, one of the most studied illnesses caused by these toxic effects is cancer since it results from a series of molecular events that change the normal cell properties. This also happens when cells are exposed to elevated levels of heavy metals as these are susceptible to redox reactions, consequently promoting the formation of reactive oxygen species (ROS). Some research has already attributed Cu toxicity to a propensity for its ions to contribute to the development of ROS, a process that transforms the structure and/or function of crucial molecules.1 Hence, this relationship is vital in order to understand carcino-genesis as a result of excessive exposure to metals since excess Cu can also induce oxidative stress by decreasing glutathione levels.7 Thus, it can be concluded that even if Cu is essential to the functioning of the human organism, it brings about problems when it is deficient and it can also lead to toxicities when in excess that could induce carcinogenesis.
Zinc (Zn) compounds exist in many objects as they are widely used by the pharmaceutical industry.8 This metal is essential not only for humans but for all living organisms as it is a constituent of about 300 enzymes and even more proteins. As a result, it plays a central role in human health given that there are numerous biological processes that require the sufficient availability of zinc.9 There is no stored form of Zn in the human body;10 it is hypothesized that the total amount of Zn in the body is 2 to 4g and the recommended daily values are approximately 8 to 11mg in the United States and 9.4 to 10mg in Europe.11
This heavy metal is also essential for the normal functioning of the immunological system and for infantile development since a deficiency in this metal is a limiting factor for growth.12 It is important for immuno-competence and it is responsible for maintaining both macrophage and neutrophil functions, as well as for the activation of natural killer cells and the phagocytic function of granulocytes.13 When this element is deficient, the mucosal barrier in the gastrointestinal tract and pulmonary tract is compromised, increasing the susceptibility to infections.9 A deficiency in Zn can also emerge in individuals who suffer from inflammatory diseases of the small intestine, renal disease, burns, and some forms of cancer, among others.13,14 Although the precise role of Zn in the regulation of apoptosis is not yet fully known, one of the origins for the loss of immunological responses is the increase in apoptosis regulated by Zn.13 Some researches have shown that Zn can either be pro- or anti-apoptotic (depending on the concentration) and that both the deprivation and excess of this element could induce an apoptotic event in the same cell line.9
Metallotheionins are zinc-binding proteins and play relevant roles in Zn-mediated immunological processes; they also act as a cellular defense against partially reduced oxygen.13 A large number of studies have established that Zn inhibits the phagocytosis and reduces the discharge of oxyradicals and the production of superoxide and hydrogen peroxide.13 However, there could even be an increase in ROS due to a G-protein coupled system activated by Zn.13 Moreover, the dislocation of this metal from zinc-binding structures (for example, finger structures in DNA repair enzymes) may be even be a more important mechanism in carcinogenesis compared to other metals that have well-described carcinogenic roles.9 A rather explored example of the involvement of Zn in cancer development is prostate cancer.9
It should be noted that Zn is one of the trace elements that participates in the foremost biological functions, depending on a complex and precise homeostatic control, on both cellular and systemic levels. Thus, its importance to the human organism is what made it an appropriate choice for discussion in this study. Nevertheless, as previously mentioned, humans do not possess any Zn stores; as a result, the dietary acquisition of this element is essential. The toxicity is rare and exceptionally fatal; however, on the other hand, the scarcity is quite frequent, especially in individuals with alimentary restrictions or chronic diseases. Based on this, Zn consumption must be in the ideal proportions as high levels of Zn can lead to carcinogenesis.
In summary, the main goal of this study is to investigate the presence or absence of these two heavy metals in samples of renal tumor tissue and to compare these levels to adjacent normal renal tissue.
Given that the renal tumor is the most well described tumor related to the exposure to heavy metals, several samples of tumor and control tissue were collected from the kidneys of seven patients (one female and six males; ages ranging from 46 to 78 years old) that were submitted for radical or partial nephrectomy. These samples were studied and compared in their chemical composition for the essential metals copper and zinc. This study was approved by the local ethics committee of Centro Hospitalar de S. João, E.P.E. and patient consent was obtained from all participants.
Electron microscopy allows the observation and characterization of large surfaces in thick samples as well as the characterization of the qualitative and semi-quantitative aspects of their chemical content. In this investigation, the Scanning Electron Microscopy was coupled to X-Ray Microanalysis (SEMXRM); this enabled images to be obtained with magnifications ranging from 10X to 500 000X. A JEOL-6301F coupled with Noren Voyager X-ray with EDS (Energy Dispersive Spectrometry) detection system was used with which it was possible to detect the presence of different heavy metals in the samples if they had a concentration above 0.2-0.3%. The samples were prepared by following an adapted protocol from Cunha et al. where the samples were fixed in a 3% glutaraldehyde solution and subsequently dehydrated in ethanol and critical point-dried in a Balzer's apparatus.15 The preparation was mounted on metal stubs and coated by carbon under a vacuum.
To our knowledge, this technique has not been used before to study the presence or absence of heavy metals in tumor tissue. This process allows one to qualitatively identify all the metals that are on the surface of the tissue. Among all of the elements detected, Cu and Zn were chosen for study because even though they are heavy metals (and thus usually considered maleficent), they are also essential to the functioning of the human organism in the appropriate concentrations, as previously mentioned.
By using the SEM-XRM technique it was possible to obtain microanalysis spectrums (qualitative and semi-quantitative) of the focused zones due to backscattered electrons, and the topographic visualization of the tissue surface due to secondary electrons. First, all the samples (tumor tissue and control samples) were processed using secondary electrons which allowed for the visualization of white spots that corresponded to the deposition of heavy metals (particles with high molecular weight) in tumor tissue samples. Then, the identification of these heavy metals in all of the opaque inclusions was made by the screening of the spots with X-ray elemental microanalysis in order to determine which of them contained heavy particles. Thus, this innovative technique allowed for the in situ identification of heavy metal particles.
Despite the limited number of patients, it was possible to test the association between the tumor and control tissues and the presence or absence of Zn and Cu using the Pearson's Chi-squared test in the R statistical software (R Development Core Team, 2011).
The patients in the study had three different types of renal cancer: clear-cell carcinoma (6 patients), and adenoma and papillary cell carcinoma (both in one patient but in two different pieces that were collected from the same kidney).
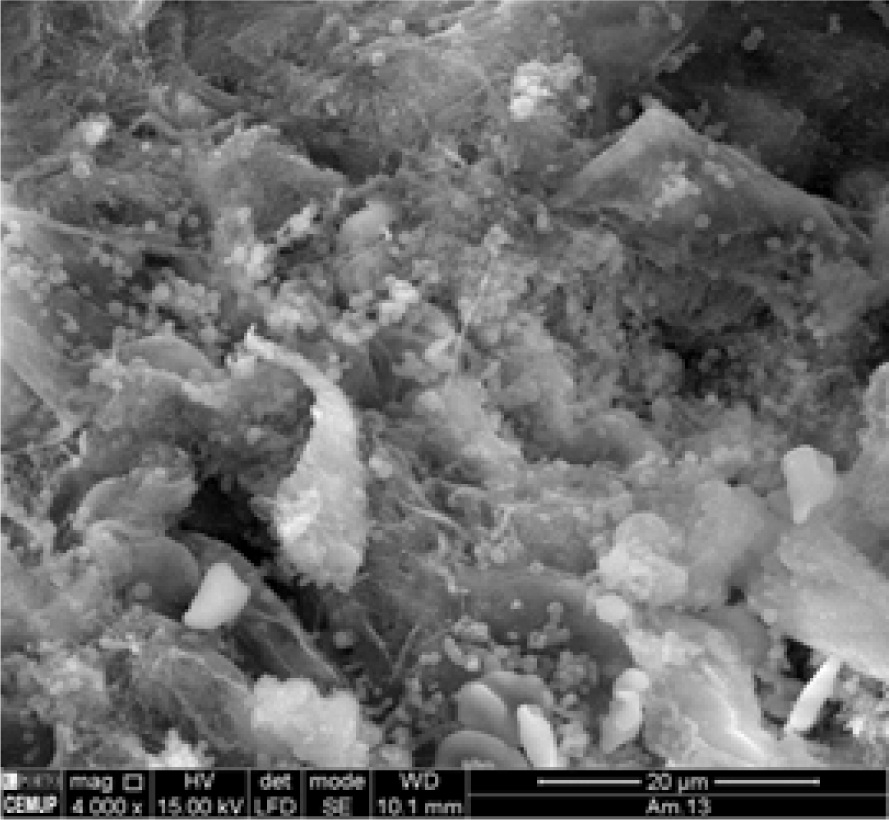
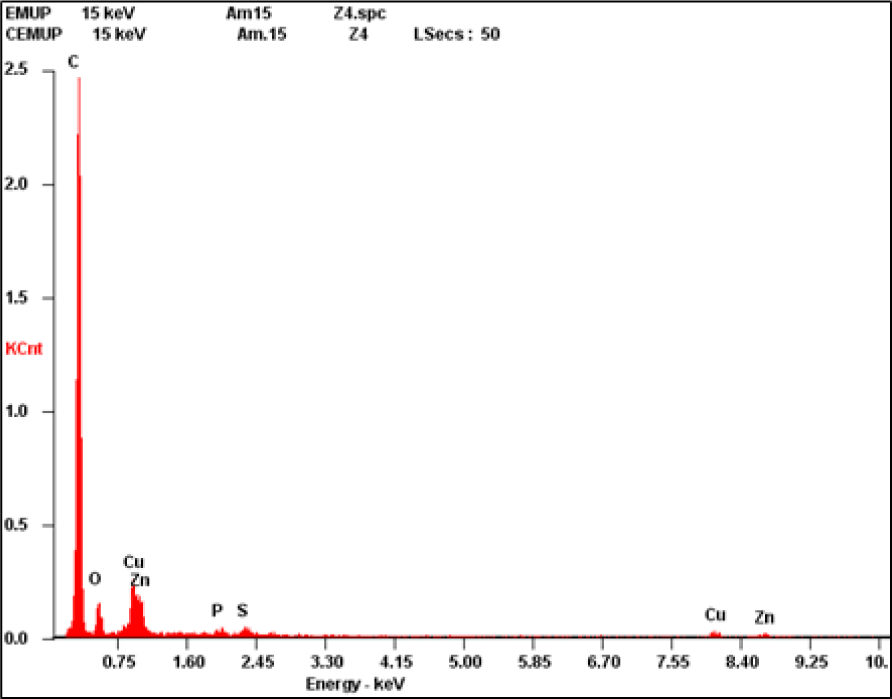
The results obtained demonstrate that the chemical composition of Cu and Zn in the tumor tissue (Figure 2A and Figure 2B) is different from the one in the control tissue (Figure 1A and Figure 1B) (2 = 10.285, df = 1, p-value = 0.0013). It was verified that Cu and Zn were present in the tumor tissue (Figure 2C) but not in the control tissue (Figure 1C).
Figure 1A.Secondary Electron Image from Control Renal Tissue. Magnification at 4000x.

Backscattered Electrons Image from Control Renal Tissue. Z2 Corresponds to the Zone where the Chemical Composition was Obtained by X-Ray Microanalysis. Magnification at 4000x.

X-Ray Microanalysis (XRM) Spectrum Showing the Absence of Metals and the Presence of Normal Elements in Control Renal Tissue (Carbon (C), Nitrogen (N), Oxygen (O), Sodium (Na), and Sulfur (S) in the Analyzed Zone Marked as Z2 in Figure 1B.
Secondary Electron Image from Renal Tumor Tissue. Magnification at 4000x.

Backscattered Electrons Image from Renal Tumor Tissue. Z4 Corresponds to the Zone where the Chemical Composition was Obtained by X-Ray Microanalysis. Magnification at 4000x.

X-Ray Microanalysis (XRM) Spectrum Showing the Presence of Copper (Cu) and Zinc (Zn) in Renal Tumor Tissue in the Analyzed Zone Marked as Z4 in Figure 2B.

The tumor tissue and the control tissue differed in their chemical composition, especially regarding the accumulation of Cu and Zn in the spot marked as Z4 in Figure 2B, which is from the tumor tissue itself. In the following graphics, there is no Cu and Zn spike from the X-Ray Microanalysis in the control sample, corresponding to Figure 1C (performed in the spot marked as Z2 in Figure 1B). This is contrary to Figure 2C where the spike can be seen. The control sample did not have these heavy metals, at least in concentrations higher than 0.2-0.3% in mass, which was not the case in the tumor samples where these heavy metals were detected.
There are numerous essential metals, including Cu and Zn, required for the necessary functioning of living organisms. However, even though they are essential for biological functions, they can become toxic in high levels. Heavy metals pose the most concern for human health because they can easily accumulate in the organism. Cadmium (Cd), mercury (Hg), lead (Pb), arsenic (As), and chromium (Cr) have already been described and classified as carcinogenic.16
It is vital that the equilibrium is maintained between free radical production and antioxidant defenses as free radicals are related to the promotion of carcinogenesis by modifying DNA and even altering the cellular antioxidant defense system.17 When the balance tends to favor the generation of these radicals, the organism is in oxidative stress where lipids, proteins, and DNA become oxidized, inhibiting their normal function and leading to several pathologies, including cancer.18
Currently, cancer is one of the diseases that affects a significant portion of the population and it represents a major concern to worldwide health systems. Cancer cells have different features depending on the type of tumor involved; this allows them to grow and metastasize to other organs. Tumor cells grow more rapidly than healthy cells, which is the reason that this disease spreads so quickly throughout the body. In most carcinoma types, there is a previous inflammatory disease that exists prior to the malign alteration. In other cancer types, an oncogenic alteration induces an inflammatory microenvironment that promotes tumor development. In 2008, Mantovani et al. published an article stating that cellular inflammatory mediators are important constituents of a tumor's local environment.18 Thus, when present, the inflammation processes help promote proliferation and tumor cell survival in addition to facilitating metastasis.18
The main goal of this investigation was to compare qualitatively the chemical content of both Cu and Zn in renal tumors and control tissue using the SEM-XRM technique. The results demonstrate that these heavy metals are present in tumor tissue but absent in control tissue (normal tissue adjacent to the tumor). Therefore, the results support a notion previously postulated by Brys et al. and Ogunlewe et al. where different metals in high levels, whether essential or not, are related to multi-organ carcinogenesis.18,19 In addition, Hardell et al., concluded that there is an increase in Cd, Zn, and Cu levels in kidneys that have cancer cells.20 Moreover, in 2013 Pirincci et al., detected high levels of Pb in renal carcinoma patients.21 However, contrary to these findings, Fassina et al., demonstrated a significant decrease in Cd and Zn concentrations in all of their studied neoplastic tissues of renal cell carcinoma.22 Karcioglu et al., showed that Cd is not found in tumor samples but it is normally present in kidney tubular cells. This study reported that Zn and Cu proportions are reduced in renal tumor tissues.23 Furthermore, Dobrowolski et al. reported low concentrations of Cd in renal cell carcinoma and low concentrations of Pb in the cortex of cancerous kidneys.24 A few years later, Cerulli et al. observed the presence of low concentrations of Cd but high Pb levels in excised tumor tissue with elevated levels of both of these two metals in the adjacent (normal) tissue.25 Importantly, the technique that was used in our study had not been used in the other related studies.
Although renal cancer is the most widely studied cancer related to heavy metal exposure, research has also examined heavy metal exposures in other types of cancer. Namely, in 2003 Waisberg et al. published a study demonstrating lung carcinoma induction when exposed to Cd.26 In 2012, Natalie et al., discovered that the chronic exposure to Cd could also lead to breast cancer.27 In addition, a study examined the association between Zn intake (as a supplement) and prostate cancer risk, in which they observed 2901 new cases of prostate cancer in 14 years.9 The risk of prostate cancer was found to be increased by the long-term supplementation of Zn with doses higher than 100 mg/day. This may not, however, be a result of the direct carcinogenicity of this metal, but rather explained by the immunosuppression provoked by the high doses of Zn.9
If our results could be confirmed by future studies, the hypothesis that heavy metals could be the cause or the consequence of the carcinoma could be further clarified. Namely, additional research may provide an answer to the question of whether these elements could be used as biomarkers with prognostic or diagnostic implications in clinical practice.
As is evident, the precise relationship between heavy metals and carcinoma is controversial and ambiguous. It is known and well described that heavy metals are involved in the carcino-genesis; this fact is widely supported in various publications. Our study is intended to highlight the presence of the essential metals Cu and Zn in tumor tissue, likely due to their accumulation over time as an anomalous sequestration of these heavy metals. However, the technique used to study these metals may be seen as a limitation as it is qualitative and only semi-quantitative.
Heavy metals are suspected to be a risk factor for the development of malignancy. But, at this time, it is unclear if these elements are either the cause or the consequence of the cancer or if they are involved in carcinogenic pathways. This work complements previous findings examining the possible relationship of essential heavy metals with carcinogenesis.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conception and design the work/idea: TN, MS, EC. Collect data/obtaining results: TN, MS. Analysis and interpretation of data: TN, MS, EC. Write the manuscript: TN. Critical revision of the manuscript: EC. Approval of the final version: EC. Contribution of patients or study material: EC. Obtaining financing: EC.
1. Gaetke LM, Chow-Johnson HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol. 2014 Nov; 88 (11): 1929–38.
2. Huang C, Chen QL, Luo Z, Shi X, Pan YX, Song YF et al Time-dependent effects of waterborne copper exposure influencing hepa tic lipid deposition and metabolism in javelin goby Synechogobius hasta and their mechanism. Aquat Toxicol. 2014 Oct; 155: 291–300.
3. Whitehouse MW, Walken WR. Copper and inflammation. Agents Actions. 1978 Jan; 8 (1-2): 85–90.
4. Bustamante JB, Mateo MC, Fernandez J, de Quiros B, Manchado OO. Zinc, copper and ceruloplasmin in arteriosclerosis. Biomedicine. 1976 Sep 30; 25 (7): 244–5.
5. Vyden JK, Throner J, Nagasawa K, Takano T, Groseth-Dittrich MF, Perlow R et al Metabolic and cardiovascular abnormalities in patients with peripheral arterial disease. Am Heart J. 1975 Dec; 90 (6): 703–8.
6. Al-Ebraheem A, Farquharson MJ, Ryan E. The evaluation of biologically important trace metals in liver, kidney and breast tissue. Appl Radiat Isot. 2009 Mar; 67 (3): 470–4.
7. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011 May 10; 283 (2-3): 65–87.
8. Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002; 21 (3-4):291–5.
9. Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010 Apr; 7 (4):1342–65.
10. Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract. 2014, 2014: 70 9152.
11. Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015 Jan; 29: 24–30.
12. Onosaka S, Tetsuchikawahara N, Min KS. Paradigm shift in zinc: metal pathology. Tohoku J Exp Med. 2002 Jan; 196 (1):1–7.
13. Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc:a multipurpose trace element. Arch Toxicol. 2006 Jan; 80 (1): 1–9.
14. Chronopoulos J, Haidouti C, Chronopoulou-Sereli A, Massas I. Variations in plant and soil lead and cadmium content in urban parks in Athens, Greece. Sci Total Environ. 1997 Mar 9; 196 (1): 91–8.
15. Cunha EM, Silva DP, Aguas AP. High-resolution identification of mer cury in particles in mouse kidney after acute lethal exposure. Biometals. 2003 Dec; 16 (4): 583–90.
16. Jennette KW. The role of metals in carcinogenesis: biochemistry and metabolism. Environ Health Perspect. 1981 Aug; 40: 233–52.
17. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008 Jul 24; 454 (7203): 436–44.
18. Bryś M, Nawrocka AD, Miekoś E, Zydek C, Foksiński M, Barecki A, et al Zinc and cadmium analysis in human prostate neoplasms. Biol Trace Elem Res. 1997 Winter; 59 (1-3): 145–52.
19. Ogunlewe JO, Osegbe DN. Zinc and cadmium concentrations in indi genous blacks with normal, hypertrophic, and malignant prostate. Cancer. 1989 Apr 1; 63 (7): 1388–92.
20. Hardell L, Wing AM, Ljungberg B, Dreifaldt AC, Degerman A, Hal mans G. Levels of cadmium, zinc and copper in renal cell carcinoma and normal kidney. Eur J Cancer Prev. 1994 Jan; 3 (1): 45–8.
21. Pirincci N, Gecit I, Gunes M, Kaba M, Tanik S, Yuksel MB et al Levels of serum trace elements in renal cell carcinoma cases. Asian Pac J Cancer Prev. 2013; 14(1): 499–502.
22. Fassina AS, Calliari I, Sangiorgio A, Rossato M, Ramigni M, Dal Bianco M et al Quantitative analysis of trace elements in human clear cell carcinoma of the kidney by energy-dispersive X-ray fluorescence. Eur Urol. 1990; 18 (2): 140–4
23. Karcioglu ZA, Sarper RM, Van Rinsvelt HA, Guffey JA, Fink RW. Trace element concentrations in renal cell carcinoma. Cancer. 1978 Sep; 42 (3): 1330–40.
24. Dobrowolski Z, Drewniak T, Kwiatek W, Jakubik P. Trace elements distribution in renal cell carcinoma depending on stage of disease. Eur Urol. 2002 Nov; 42 (5): 475–80.
25. Cerulli N, Campanella L, Grossi R, Politi L, Scandurra R, Soda G et al Determination of Cd, Cu, Pb and Zn in neoplastic kidneys and in renal tissue of fetuses, newborns and corpses. J Trace Elem Med Biol. 2006; 20(3): 171–9.
26. Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003 Nov 5; 192 (2-3): 95–117.
27. Aquino NB, Sevigny MB, Sabangan J, Louie MC. The role of cadmium and nickel in estrogen receptor signaling and breast Cancer: metalloestrogens or not? J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012; 30(3): 189–224.
Tânia Nogueira, 1 Universidade de Vigo, Spain.
Mariana Semedo, 2 Instituto de Ciências Biomédicas Abel Salazar, Portugal.
Elisabete Cunha, 3 Faculdade de Medicina da Universidade do Porto, Portugal.
About the Author: Elisabete Cunha, MD, PhD is currently a first year intern at Hospital de São João in Porto, Portugal.
Correspondence Tânia Nogueira. Address: Lagoas s/n, 36310 Vigo, Pontevedra, Spain. Email: taniambnog@gmail.com
Cite as: Nogueira T, Semedo M, Cunha E. Essential Heavy Metals in Renal Tumor Tissue and Its Possible Relation to Carcinogenesis: Applying the Scanning Electron Microscopy Coupled with X-Ray Microanalysis Technique. Int J Med Students. 2014 Nov-2015 Mar;3(1):10-4.
Copyright © 2015 Tânia Nogueira, Mariana Semedo, Elisabete Cunha
International Journal of Medical Students, VOLUME 3, NUMBER 1, March 2015