Prevalence of Neural Tube Defects (NTD) per 10,000 Live Births in the United States from 1995-2011.
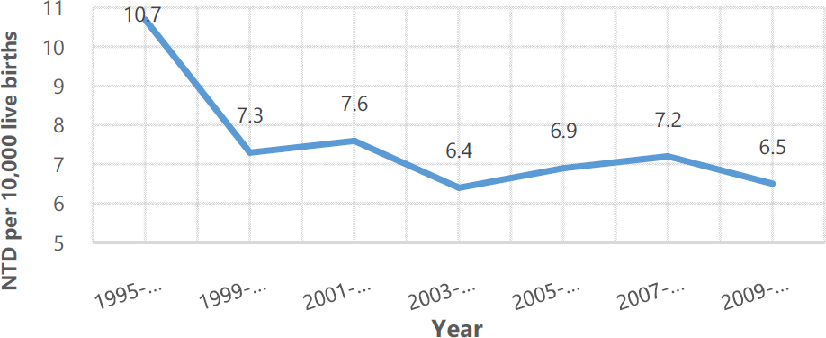
Legend: Neural tube defects defined as both spina bifida and anencephaly. Mandatory folic acid fortification introduced in 1998.5
Natalie C. Campbell1, Stephen B. Trippel2, Eric A. Nauman3
doi: http://dx.doi.org/10.5195/ijms.2022.1127
Volume 10, Number 4: 405-412
Received 26 07 2021; Rev-request 08 08 2021; Rev-request 13 02 2022; Rev-recd 09 09 2021; Rev-recd 02 04 2022; Accepted 11 04 2022
ABSTRACT
Spina bifida is a neural tube defect resulting from an incomplete closure of the caudal neuropore. The most debilitating form of spina bifida, myelomeningocele (MMC), can present with Chiari II malformation with concomitant hydrocephalus, bowel and bladder abnormalities, and impaired motor function of the lower limbs. The incidence rate of spina bifida is 3.4 per 10,000 live births reported within the US. Advancements in the standard therapy, namely prenatal intervention pioneered by the Management of Myelomeningocele Study (MOMS), have aimed to reduce maternal and fetal complications, and yet complications were increased, calling for the need of further improvements. Beyond current standard interventions for MMC, the most promising developments have employed various biomedical methods ranging from isolated stem cell injections to biodegradable scaffold patches. These scaffolds can be biologic or synthetic and are often incorporated with bioactive proteins or stem cells. This review discusses the benefits and limitations of post-MOMS era biomedical engineering intervention articles found in 3 medical and biomedical databases consisting of systematic reviews, meta-analyses, randomized control trials, and experimental studies. After analysis of the advancements and limitations of these studies, an engineered synthetic biodegradable scaffold seeded with bioactive proteins and stem cells create a superior scaffold possessing watertight impermeability and cytocompatibility for successful coverage and host integration with minimal inflammation. Coupled with minimally invasive intra-amniotic injection delivery, an earlier mitigation could further prevent progression of poor neurologic outcomes, and possibly even regenerate neuronal tissue in patients with MMC.
Keywords: Myelomeningocele, Fetoscopic Surgery; Tissue Engineering; Tissue Scaffolds; Neural Tube Defect (Source: MeSH-NLM).
Spina bifida is a type of neural tube defect (NTD) caused by the failure of the caudal neuropore to close prior to day 27 of gestation.1 Failure of neural tube closure results in incomplete fusion of the vertebral arches, most commonly in the lumbar and sacral regions, allowing various amounts of central nervous system contents to expand beyond the vertebral canal.1 The incidence rate of all neural tube defects is 3.4 per 10,000 live births reported within the United States, with a 1-year survival rate of 88-96% and a survival rate into adulthood of 75%.2 The worldwide incidence rate of neural tube defects is 140,000 annually.3
The most debilitating form of spina bifida is myelomeningocele (MMC), characterized by the presence of both meninges and spinal cord outside of the vertebral canal. MMC can often present with Chiari II malformation and accompanying hydrocephalus, bowel and bladder abnormalities, and lower extremity motor function deficits. These sequelae are due to the spinal cord’s unnatural exposure to fetal waste products in amniotic fluid as well as leakage of cerebrospinal fluid (CSF).4 MMC has severe impacts on several aspects of quality of life, such as physical, psychological, social, and neurocognitive functioning. The average lifetime cost for a general MMC patients is $560,000 in 2007, estimated in 2021 to be around $722,400, highlighting the financial burden on a person living with MMC for lifelong services such as skilled caretakers and loss of ability for employment.3
Although the prevalence has decreased through prenatal folic acid supplementation and fortification of foods, preventative measures have not eradicated neural tube defects (Figure 1).5
Figure 1.Prevalence of Neural Tube Defects (NTD) per 10,000 Live Births in the United States from 1995-2011.

The current treatment of MMC was established through a well-known clinical trial titled “Management for Myelomeningocele Study” (MOMS), which spanned from 2003-2010.6 This interventional randomized study compared the previous standard treatment of 48-hour postnatal repair surgery to the then novel prenatal surgery using open surgical interventions with the placement of either an autologous dura mater graft or synthetically derived collagen matrix (DuraGen)6 during weeks 19-25 of gestation.6 The trial found that the prenatal open surgical intervention reduced neonatal death or the need for ventricle shunt placement, and improved motor function as well as quality of life outcomes of fetuses with MMC (Table 1).6
Table 1.MOMS Statistically Significant Outcomes Comparison between the Prenatal and Postnatal (control) Cohorts at 12 Months Postnatal for Primary Outcomes and 30 Months for Secondary Outcomes.
| Prenatal Surgery | Postnatal Surgery (Control) | p-value | |
|---|---|---|---|
| Primary Infant Outcomes at 12 Months | |||
| Shunt criteria met | 65% | 92% | 0.001* |
| Placement of shunt | 40% | 82% | 0.001 |
| Any hindbrain herniation | 64% | 96% | 0.001 |
| Any brainstem kinking | 20% | 48% | 0.001 |
| Abnormal location of fourth ventricle | 46% | 72% | 0.002 |
| Syringomyelia | 39% | 58% | 0.03 |
| Difference between motor function and anatomical level | 0.58±1.94 | ‒0.69±1.99 | 0.001† |
| Secondary Outcomes of Children at 30 Months | |||
| Mean Bayley Psychomotor Development Index | 64.0±17.4 | 58.3±14.8 | 0.03‡ |
| Peabody stationary score | 7.4±1.1 | 7.0±1.2 | 0.04§ |
| Peabody locomotion score | 3.0±1.8 | 2.1±1.5 | 0.002§ |
| Peabody object manipulation score | 5.1±2.6 | 3.7±2.1 | 0.001§ |
| Walking independently on examination | 42% | 21% | 0.01 |
| Walking status | 0.03 | ||
| No walking ability | 29% | 43% | |
| Walking with orthotics/devices | 29% | 36% | |
| Walking without orthotics/devices | 42% | 21% | |
| WeeFIM self-care score | 20.5±4.2 | 19.0±2.4 | 0.02¶ |
| WeeFIM mobility score | 19.9±6.4 | 16.5±5.9 | 0.003¶ |
Based on this data, open surgical intervention has become the standard treatment of MMC. Open fetal surgery did not come without its cost of significantly increased fetal and maternal adverse outcomes. (Table 2)6 Statistically significant maternal outcomes included oligohydramnios, pulmonary edema, blood transfusion, chorionic membrane rupture, spontaneous membrane rupture, and spontaneous preterm labor. Statistically significant fetal outcomes included bradycardia during intervention, gestational age at birth, mean birth weight, and respiratory distress syndrome.6
Table 2.Comparing Both Statistically Significant Maternal and Fetal Outcomes Arising from Prenatal vs Postnatal Surgery.
| Outcomes | Prenatal Surgery | Postnatal Surgery (control) | p-value |
|---|---|---|---|
| Maternal Outcomes | |||
| Blood transfusion at delivery | 9% | 1% | 0.03 |
| Chorionic membrane separation | 26% | 0% | 0.001 |
| Spontaneous membrane rupture | 46% | 8% | 0.001 |
| Spontaneous labor | 38% | 14% | 0.001 |
| Oligohydramnios | 21% | 4% | 0.001 |
| Placental abruption | 6% | 0% | 0.03 |
| Pulmonary edema | 6% | 0% | 0.03 |
| Fetal outcomes | |||
| Gestation age at birth, weeks | 34.1±3.1 | 37.3±1.1 | 0.001 |
| Bradycardia during fetal or neonatal repair | 10% | 0% | 0.003 |
| Mean birth weight, grams | 2383±688 | 3039±469 | 0.001 |
| Respiratory distress syndrome | 21% | 6% | 0.008 |
In response to these adverse outcomes, engineered scaffold patches have been proposed to serve as advanced treatment for replacement of lost tissue and an earlier alternative for wound closure as fetal tissues cannot easily be manipulated before 19 weeks.7 Additionally, minimally invasive methods have been proposed to lower surgical complications in both the fetus and mother when compared to open fetal surgery.8
The purpose of this narrative review is to provide a clinical perspective in the various methods (Table 3), results, limitations, and future implications of research in the post-MOMS era, and how it has progressed the prenatal intervention of MMC to provide earlier intervention in MMC repair while minimizing both maternal and fetal peri-operative complications. More specifically, this narrative review serves as a novel approach to compare not only scaffold compositions, but also effects of various bioactive proteins seeded scaffolds, and method of administration.
Table 3.Summary of the Biomedical Engineering Study Methods Used by Various Groups to Improve Upon Standard Treatment of Myelomeningocele in the Post-MOMs Era.
| Literature | Subjects | Method of MMC creation | Nature vs Synthetic scaffold | Scaffold material | Seeded with bioactive proteins | Seeded with stem cells | Method of intervention |
|---|---|---|---|---|---|---|---|
| Biologic scaffolds seeded with bioactive proteins | |||||||
| Watanabe et al.9 | Rats | RA induction | Natural | Gelatin | bFGF | – | Open fetal surgery |
| Watanabe et al.10 | Rats | RA induction | Natural | Gelatin microspheres | – | – | Intra-amniotic injections |
| Watanabe et al.11 | Sheep | Surgical creation | Natural | Gelatin sponge | bFGF | – | Open fetal surgery |
| Biologic scaffolds seeded with stem cells | |||||||
| Brown et al.13 | Sheep | Surgical creation | Natural | Amniotic membrane | – | Early and late gestational pMSCs | Open fetal surgery |
| Li et al.15 | Rats | RA induction | Natural | Chitosan-gelatin | – | BMSCs | Open fetal surgery |
| Isolated stem cell intervention | |||||||
| Dionigi et al.16 | Rats | RA induction | – | – | – | afMSCs | Intra-amniotic injections |
| Feng et al.17 | Rats | RA induction | – | – | – | afMSCs, pMSCs | Intra-amniotic injection |
| Synthetic biodegradable scaffolds | |||||||
| Oria et al.19 | Rats | – | Synthetic | PLA, PCL | – | – | Subcutaneous and dural implantation |
| Tatu et al.21 | – | – | Synthetic | PLA, PCL | – | – | – |
Articles for this narrative review were selected from 1 June 2019 – 31 July 2020 utilizing a key word search via PubMed, Science Direct, Online Wiley Library, Via Medica. Key words included: “Myelomeningocele”, “Fetal Surgery”, and “Tissue Engineering”. Articles included ranged from publication in 2010-2019.
Inclusion criteria included articles published in the last 10 years; specific types of literature: review articles, clinical trials, meta-analyses, randomized control trials systematic reviews, and book chapters; in-vivo and in-vitro techniques, surgical or chemical creation of MMC.
Exclusion criteria included specific type of literature: conference abstracts, correspondences, encyclopedias, discussions, editorials, news, short communications, and literature categorized as “other”; literature describing a second surgery for device retrieval; literature largely discussing broad techniques in fetal surgery; literature largely discussing topics outside scope of this review: i.e., urology, urodynamics, obstetrics and gynecology, general birth defects, and perioperative techniques.
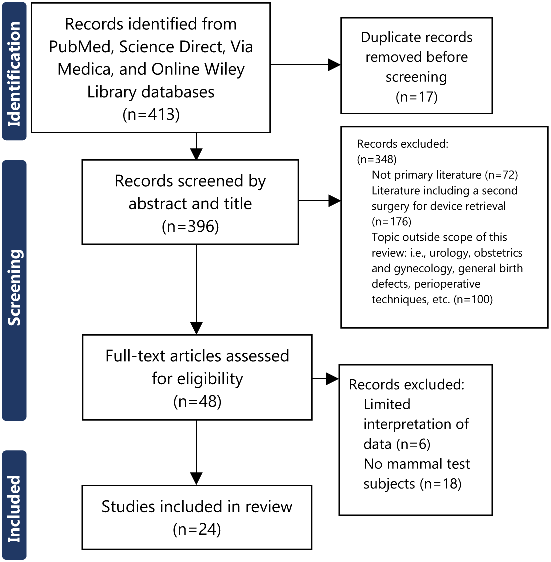
Since the MOMS trial, tissue engineering studies have been conducted through various avenues. A total of 413 pieces of literature were identified with ultimately 24 meeting the inclusion criteria (Figure 2).
Figure 2.PRISMA Study Flowchart.
A natural biological scaffold is uniquely derived from an existing organic source. Popular organic sources have included bovine, ovine, and porcine bone that researchers have ultimately transformed into gelatin, collagen, or a hybrid of the two.9–14,17–19 The porous nature of the scaffold materials allows for scaffold-host integration via cellular differentiation and neovascularization, resulting in defect coverage as well as similar innate mechanical properties of native tissue.9–14,17–19 A natural biological scaffold benefits from being biodegradable, and thus, not requiring a future surgery for its removal. Scaffolds can also serve as vessels to deliver bioactive proteins, stem cells, and other small molecules.
Watanabe et al. conducted a series of 3 studies exploring the utility of natural biological scaffolds seeded with bioactive proteins.9–11 They tested gelatin-based sponges seeded with basic fibroblast growth factor (bFGF) using chemically induced (via retinoic acid) MMC fetal rats in their 2010 and 2011 studies, and then, surgically induced MMC fetal sheep in their 2016 study. The group chose bGFG for its ability to induce both epithelization and neovascularization, and gelatin for its porosity together promoting host integration. The methods of scaffold placement differed: 2010 study via open fetal surgery, 2011 via ultrasound-guided intra-amniotic injections, and 2016 via open fetal surgery.
In 2010, Watanabe et al. showed enhanced incorporated epithelialization and neovascularization, with the seeded bFGF gelatin scaffolds compared to the untreated control subjects.9 A total of 32 surviving fetal rats were analyzed, showing that both epidermal ingrowth and neovascularization were significantly greater in bFGF seeded cohort compared to non-seeded cohort, (p<0.05) highlighting the clear benefit of bFGF.9 One limitation in this study included early degradation of the sponges resulting in partial defect coverage. This study improved upon the MOMS trial as it exemplifies that a porous patch can support neovascularization and epithelialization, enhanced by bFGF in place of suture closure.
In 2011, Watanabe et al. continued their study using biologic scaffolds (via gelatin microspheres) with a shift in focus on using intra-amniotic injection therapy.10 This method highlighted a major benefit to treatment intervention as injection therapy does not rely on tissue strength as much as suture, thus allowing for earlier gestational intervention. A total of 52 surviving fetal rats were analyzed with the injectable microsphere cohort measuring significantly greater epidermal thickness compared to group without intervention (p < 0.05).10 One limitation was using rats as they have relatively short gestations. This study improved upon the MOMS trial by offering injectable therapy as a potential method for earlier intervention compared to the standard open surgical approach.
In 2016, Watanabe et al. applied their biologic scaffold to a large animal study.11 This sheep model allowed the group to observe the subjects longer (average gestation of sheep: 20–22 weeks) and allowed for better theorized application to the human counterpart. Additional analysis in this 2016 study measured preservation of spinal cord material and the degree of hindbrain herniation in the 5 surviving fetal sheep separated into unique cohorts, with varying component-seeded gelatin scaffolds. Results showed the experimental groups, with preserved spinal cord material through significantly greater tissue thickness overlying the spinal cord, compared to group without intervention (p < 0.0001).11 Additionally, hindbrain herniation in the experimental groups was significantly less when compared to the control group (p <0.01).11 Limitations of this study included inconsistent surgical MMC creations with unknown effects of innate epithelial healing vs scaffold-mediated neoepithelialization. This large animal study allowed Watanabe et al. to get closer to translational human studies and applying the post-MOMs trial advancements discussed in their 2010 and 2011 studies.
One unique utility of stem cells is their pluripotency, the capacity to become several cell derivatives. Stem cells have the potential to influence MMC coverage not only with spinal cord protection via epithelization, but also with an additional role in neuron regeneration. Due to the prolonged spinal cord exposure to the harmful fetal waste products in amniotic fluid, motor neuronal death leads to the observed lower motor extremity deficits seen in postnatal subjects.12 The idea behind seeding natural biological scaffolds with stem cells has the potential to serve two important functions: cover the MMC defect reducing further neurological damage, while also promoting regeneration of motor neurons in the spinal cord.
In 2016, a study by Brown et al., compared the utility of autologous amniotic membrane patches seeded with early gestational placenta-derived mesenchymal stem cells (pMSCs) vs late gestational pMSCs in surgically created MMC fetal sheep.12 The authors noted that early gestational pMSCs has been found to produce factors associated with neural protection.13 The cohorts were compared using histological analysis of the lambs’ spinal cords after birth and neurological testing focusing on limb movement, stance, hindlimb weight bearing, standing, stepping, and hindlimb clearance.14 The early gestation pMSC seeded cohort showed the greatest proportion of defect coverage, as well as normal ambulation compared to the lower motor neuron dysfunction in late gestation pMSCs.12 Limitations of this study included limited statistical analyses, as well as the subjective use of the motor function scale. Brown et al.’s study illustrates the potential benefit of incorporation of early gestational pMSCs, highlighting its convenient autologous nature as well as adjunct immunomodulating cytokines and neuronal protection.
In 2016, Li et al. engineered a chitosan-gelatin scaffold seeded with bone marrow mesenchymal stems cells (BMSCs) applied using microsurgery technology to analyze its efficacy in patching defects and regenerating neurons in chemically created MMC in fetal rats.15 The authors chose chitosan-gelatin scaffolds for its high composition of collagen, lack of antigenicity, and large pore size of 300µm, all important qualities to facilitate cell growth and metabolism.15 BMSCs were chosen for their angiogenesis and ability to prevent fibrosis.15 The study found that the transplanted BMSCs seeded chitosan-gelatin scaffolds lessened MMC defects as well as expressed markers of neural stem cell and neurons.15 Some notable limitations to this study were the absence of statistical analysis, as well as the late treatment application. The results from Li et al.’s study show successful alternative materials for tissue scaffolds, highlighting high porosity of gelatin being able to support both tissue repair and regeneration aided with adjunct BMSCs seeding.
Isolated stem cell injections do not have the same size and space occupations as biological scaffolds and consequently they can be administered by tools as small as needles. Some of the benefits of injectable therapy with stem cells not only includes the already mentioned pluripotency of stem cell and its autologous nature, but this type of therapy also opens the opportunity for earlier therapy, and lower surgical complications.16,17
In 2015, Dionigi et al. tested the effect of trans-amniotic stem cell therapy (TRASCET) with amniotic fluid mesenchymal stem cells (afMSCs) on chemically induced MMC fetal rats.16 This study measured the degree of brainstem and cerebellar herniation using histology and high-resolution magnetic resonance imaging in 62 fetal rats. They found that the intra-amniotic injected afMSCs cohort showed less brainstem and cerebellar herniation as well as more MMC coverage on histological analysis when compared to the cohort without intervention (p<0.001).16 A notable limitation of this study was the small window (1 week) between induced MMC and therapeutic intervention.16 The results from this study showed the potential of using TRASCET to benefit subjects with MMC utilizing a minimally invasive technique, earlier intervention, and use of autologous afMSCs in reducing neurological sequelae.
In 2016, Feng et al. compared autologous placenta-derived mesenchymal stem cells (pMSCs) and amniotic fluid-derived mesenchymal stem cells (afMSCs) via TRASCET to evaluate defect coverage in chemically induced in MMC fetal rats.17 The selection of pMSCs to compare against afMSCs was influenced by availability of prenatal testing via chorionic villus sampling (CVS) for pMSCs (10 weeks gestation) vs amniocentesis to acquire afMSCs (15 weeks gestation). The amount of coverage was compared using histological analysis of 238 fetal rats. There was no significant difference in complete defect coverage between the afMCSs and pMSCs cohorts or when both were compared to the cohort without intervention.17 A limitation of this study was the lack of analysis apart from reporting the amount of defect coverage. The results from this study illustrate that earlier acquirement of pMSCs via CVS vs afMSCs via amniocentesis do not appear to aid in earlier MMC defect coverage.
Most synthetic biodegradable scaffolds are broadly characterized as non-toxic, biodegradable, easily reproducible, and resist early destructive enzymatic breakdown.18 Synthetic scaffolds can have a self-expanding quality in body temperature, ideal for achieving complete coverage of the MMC defect starting with a small injectable product.19–21
In 2019, Oria et al. studied the in-vivo effects of a blended PLA (poly l-lactic acid) and PCL (poly ε-caprolactone) biodegradable synthetic scaffold via subcutaneous and dural implantation in anatomically normal rats.19 Tissue analysis of the PLA-PCL group revealed no signs of neural inflammation via absence of astrocytic reaction or glial scar formation.19 A limitation of this study was that the patch was not directly tested using animal MMC models and its effect in prenatal treatment. The biocompatible results from this study support integration of biodegradable synthetic scaffolds for MMC interventions.
In 2019, Tatu et al. also studied biodegradable PLA and PCL blended synthetic scaffolds with focus on its characteristics; namely the scaffold’s in-vitro self-expansion, permeability, and biodegradable abilities.21 The patch was observed in-vitro to self-expand at body temperature (37° C), impermeable to water, and did not degrade while studied in amniotic fluid.21 One limitation of the study was the properties of their biodegradable synthetic patch were not tested in-vivo, limiting application to MMC treatment. Based on their in-vitro studies, synthetic scaffolds have several favorable properties that could make it a useful alternative in MMC repair.
Currently, there are sparse systematic reviews or meta-analyses that consider the material of scaffolds as well as any seeded materials for earlier and safer intervention for MMC therapy. One meta-analysis by Kunpalin et al. focuses on the efficacy of stem cell injections as well as stem cell seeded biologic scaffolds but does not include discussion of synthetic scaffolds or the use of bioactive proteins.22 This narrative review serves as a novel approach to compare not only scaffold compositions, but also effects of various bioactive proteins seeded scaffolds, and method of administration.
After analyzing the contributions and limitations of these studies, a combination of different materials and methods could theoretically produce a patch that can successfully prevent and potentially reverse poor neurological outcomes in patients with MMC. It is unclear which source of stem cells and selection of bioactive proteins would be most beneficial to serve this role as no existing study compares them directly. However, the successes in the various studies mentioned in this review suggest there could be multiple solutions.
When comparing biological versus synthetic scaffolds, synthetic scaffolds are superior for several reasons. Biodegradable synthetic scaffolds could mitigate some limitations of biologic scaffolds. Biologic scaffolds have the potential to initiate an immune response, due to its antigenicity, which in turn could interfere with other biological processes, such as general development, tissue healing and tissue regeneration.23 Synthetic biodegradable materials lack antigenicity, and thus have less risk of producing an immune reaction. Synthetic scaffolds also have greater mechanical strength compared to biological scaffolds as synthetic materials are less susceptible to early degradation via host enzymatic reactions, thus preserving tensile strength to support tissue remodeling.21 Additionally, synthetic scaffolds can be designed to have large enough pores for neovascularization and epithelialization without having an open connection through the scaffold, thus prohibiting further neurologic degradation via amniotic fluid and preventing progressive CSF loss.19 The self-expansion characteristic of the synthetic patch can decrease operative time, potentially decreasing fetal and maternal operative-related complications.21
Regarding selection of bioactive protein seeding, it is clear from the studies discussed in this paper that bFGF has a favorable effect on epithelialization and neovascularization ultimately providing host integration and neurologic protection.9 Additionally, selection for early placenta-derived mesenchymal stem cells has also shown to aid in MMC defect coverage and hopeful neural regenerative properties, as demonstrated in the study conducted by Brown et al.13 The benefit of its autologous nature and early retrieval through chorionic villus sampling beginning at 10 weeks’ gestation allow pMSCs to be a convenient and a potential restorative seeding material.
Upon analyzing the various delivery methods used in these studies, an in-utero injectable approach could be superior as it allows for less invasive and earlier intervention compared to the current treatment of open fetal surgery.24 It is unclear how early an injectable delivery method could be implemented in humans, and future research into this area is required, but the goal of intervention should be as close to MMC diagnosis (typically gestational week 16–18) as possible.
Finally, it is important to consider the limitations of the discussed studies to help avoid future pitfalls. Some suggestions for future studies using the methods proposed in this discussion should include larger cohorts, use of large animals with gestation lengths closer to humans, and measurement of outcomes like that of the MOMS trial for greater assessment of a study’s advancements on current treatment of MMC.
Since the MOMS era spanning from 2003–2010, great emphasis has been placed on engineering a scaffold that can preserve and possibly reverse the neurological deficits seen in patients with myelomeningocele in a way that poses minimal risk to the health of the mother and baby. Scientists have gone down several unique avenues to offer therapeutic solutions for earlier and safer intervention, yet there is no clear superior intervention at this time. Upon analysis of the advancements and limitations of several studies, patients with MMC defects could benefit from an engineered synthetic biodegradable scaffold seeded with bFGF and placenta-derived mesenchymal stem cells. This combination would aim to incorporate the qualities that many studies have highlighted as crucial for an MMC scaffold to possess. Delivery of this scaffold would ideally be placed via intra-uterine injection(s) shortly after diagnosis of MMC. Not only could this solution serve to prevent poor neurological outcomes caused by MMC, but it could reduce the healthcare cost of multiple surgeries, hospitalizations, and lifestyle adjustments associated with the current MMC therapies.
Biomedical Engineering Advancements after Management of Myelomeningocele Study (MOMS): A Narrative Review
Spina Bifida is birth defect in the spine that can cause damage to the spinal cord before a baby is born. There are several types of spinal bifida, each with varying degrees of spinal cord damage. There is also a range of complications from damaging the spinal cord including hydrocephalus (water on the brain), bowel and bladder incontinence, and impaired leg mobility. Historically, the standard of care to treat myelomeningocele (one of the more severe types of spina bifida) involved surgery shortly after birth. As science has advanced, a large study called MOMS “Management of Myelomeningocele Study” spanning from 2003-2010 compared the number of complications between repairing spina bifida defects while the baby was still in the womb with the standard method of postnatal repair. The MOMS trial found less complications in both the moms and the babies in the prenatal group when compared to the postnatal group, resulting in the new prenatal standard of care for myelomeningocele standard of care. Our group chose to research studies from 2010-2019 that aimed to further improve upon the MOMS trial with emphasis on using biomedical engineered patches that could be used to repair spina bifida defects even earlier and with less invasive methods.
Our group searched through 413 pieces of literature, using the following inclusion criteria: primary literature published within the last 10 years, with key words of ‘myelomeningocele’, ‘fetal surgery’, and ‘tissue engineering’, and using animals with lab-inflicted myelomeningocele. Studies that were excluded were certain types of primary literature such as abstracts, correspondence, encyclopedias, discussions, editorials, news, short communications, and literature categorized as ‘other’, studies that had a second prenatal surgery to remove the engineered spinal patch, as well as literature that was outside the scope of this narrative review such as studies with a primary focus on urology, obstetrics and gynecology, general birth defects, and various operative techniques. In the end, 24 studies were included in this narrative review.
Some of the differences found between the studies included method of inducing spina bifida (either chemically or surgically), the type of animal (rats vs sheep), material used for the scaffold, seeded material (proteins and or stem cells), the method of introducing the scaffold, and finally the week of gestation the scaffold was placed.
Retinoic acid was used to chemically induce some of the test subjects, while a surgical incision was used to induce spina bifida in others.
Rats were used in some studies as a more convenient and cost-effective animal subject compared to sheep; however, the consequence of using rats included shorter gestational periods compared to sheep and shorter time between induction of spina bifida and implementing the engineered scaffold. Conversely in sheep with longer gestational periods, studies were able to make stronger conclusions to human implications.
Several studies experimented with reverse engineering scaffold patches made from cow, pig, or sheep bone into a more malleable/porous collagen material. Other studies derived a similar product using synthetic materials, like PLA (poly l-lactic acid) and PCL (poly- ε caprolactone). Both biological and synthetic materials were able to serve as vessels to carry signaling proteins, naturally found in the body, to enhance incorporation of the engineered patch to the fetus prevent further damage to the spinal cord. Largely, these signaling proteins enhanced growth of nerve cells and connective tissue cells. Some studies also incorporated stem cells into their scaffolds, allowing the potential of multiple cell types to grow. All patches discussed in this review were biodegradable preventing a second surgery to retrieve the device.
The various methods of scaffold placement used were open surgery and intra-amniotic injections. The choice of method depended on several factors including gestational age, as early fetuses do not have the structural integrity to undergo open surgery and patch fixation.
Since the MOMS era spanning from 2003–2010, great emphasis has been placed on engineering a scaffold that can preserve and possibly reverse the complications seen in patients with myelomeningocele in a way that poses minimal risk to the health of the mother and baby. Scientists have gone down several avenues to offer earlier and safer intervention, yet there is no clear superior intervention at this time. Upon analysis of studies included in this narrative review, patients with myelomeningocele could benefit from an engineered synthetic biodegradable scaffold seeded with both proteins and stems cells to promote scaffold incorporation and structural integrity. Delivery of this scaffold would ideally be placed via intra-uterine injection(s) shortly after diagnosis of spina bifida. Not only could this solution serve to prevent common complications of spina bifida, but it could reduce the healthcare cost of multiple surgeries, hospitalizations, and lifestyle adjustments associated with the current therapies.
None.
The Authors have no funding, financial relationships, or conflicts of interest to disclose.
Conceptualization: NC & EN. Data Curation: NC. Formal Analysis: NC. Funding Acquisition: NC. Investigation: NC. Methodology: NC. Project Administration: NC. Resources: NC. Software: NC. Supervision: NC & ST. Validation: NC & ST. Visualization: NC. Writing - Original Draft Preparation: NC. Writing - Review and Editing: NC & ST.
1. Rochelle D, Frando M, Voto H, Baron K. Pediatric Rehabilitation. In: Maitin IB, Cruz E, editors. CURRENT Diagnosis & Treatment: Physical Medicine & Rehabilitation Eds. New York: McGraw-Hill; 2014.
2. Moldenhauer JS, Flake AW. Open fetal surgery for neural tube defects. Best Practice & Research Clinical Obstetrics & Gynaecology. 2019;58:121–32.
3. Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nature reviews Disease primers. 2015;1(1):1–8.
4. Castillo J, Lupo PJ, Tu DD, Agopian AJ, Castillo H. The National Spina Bifida Patient Registry: A Decade’s journey. Birth defects research. 2019;111(14):947–57.
5. Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR. Morbidity and mortality weekly report. 2015;64(1):1.
6. Adzick NS, Thom EA, Spong CY, Brock III JW, Burrows PK, Johnson MP, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. New England Journal of Medicine. 2011 Mar 17;364(11):993–1004.
7. Adzick N. S. (2012). Fetal surgery for myelomeningocele: trials and tribulations. Isabella Forshall Lecture. Journal of pediatric surgery, 47(2), 273–81.
8. Moldenhauer JS, Adzick NS. Fetal surgery for myelomeningocele: After the Management of Myelomeningocele Study (MOMS). In Seminars in Fetal and Neonatal Medicine. 2017; 22(6), 360–6.
9. Watanabe M, Jo JI, Radu A, Kaneko M, Tabata Y, Flake AW. A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Engineering Part A. 2010;16(5):1645–55.
10. Watanabe M, Li H, Roybal J, Santore M, Radu A, Jo JI, et al. A tissue engineering approach for prenatal closure of myelomeningocele: comparison of gelatin sponge and microsphere scaffolds and bioactive protein coatings. Tissue Engineering Part A. 2011;17(7-8):1099–110.
11. Watanabe M, Li H, Kim AG, Weilerstein A, Radu A, Davey M, et al. Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of Myelomeningocele. Biomaterials. 2016;76(1):133–43.
12. Brown EG, Saadai P, Pivetti CD, Beattie MS, Bresnahan JC, Wang A, et al. In utero repair of myelomeningocele with autologous amniotic membrane in the fetal lamb model. J Pediatr Surg. 2014; 49(1):133–8.
13. Brown EG, Keller BA, Lankford L, Pivetti CD, Hirose S, Farmer DL, et al. Age does matter: a pilot comparison of placenta-derived stromal cells for in utero repair of myelomeningocele using a lamb model. Fetal diagnosis and therapy. 2016;39(3):179–85.
14. Brown EG, Keller BA, Pivetti CD, Sitkin NA, Farmer DL, Bresnahan JC: Development of a locomotor rating scale for testing motor function in sheep. J Pediatr Surg. 2015;50(1):617–21.
15. Li X, Yuan Z, Wei X, Li H, Zhao G, Miao J, et al. Application potential of bone marrow mesenchymal stem cell (BMSCs) based tissue-engineering for spinal cord defect repair in rat fetuses with spina bifida aperta. Journal of Materials Science: Materials in Medicine. 2016;27(4):77.
16. Dionigi B, Brazzo III JA, Ahmed A, Feng C, Wu Y, Zurakowski D, et al. Trans-amniotic stem cell therapy (TRASCET) minimizes Chiari-II malformation in experimental spina bifida. Journal of pediatric surgery. 2015;50(6):1037–41.
17. Feng C, Graham CD, Connors JP, Brazzo III J, Zurakowski D, Fauza DO. A comparison between placental and amniotic mesenchymal stem cells for transamniotic stem cell therapy (TRASCET) in experimental spina bifida. Journal of pediatric surgery. 2016;51(6):1010–13.
18. Annor AH, Tang ME, Pui CL, Ebersole GC, Frisella MM, Matthews BD, et al. Effect of enzymatic degradation on the mechanical properties of biological scaffold materials. Surgical endoscopy. 2012;26(10):2767–78.
19. Oria M, Tatu RR, Lin CY, Peiro JL. In vivo evaluation of novel PLA/PCL polymeric patch in rats for potential spina bifida coverage. Journal of surgical research. 2019;242:62–9.
20. Matta AK, Rao RU, Suman KN, Rambabu V. Preparation and characterization of biodegradable PLA/PCL polymeric blends. Procedia materials science. 2014;6(1):1266–70.
21. Tatu R, Oria M, Pulliam S, Signey L, Rao MB, Peiro JL, et al. Using poly (l-lactic acid) and poly (ε-caprolactone) blends to fabricate self-expanding, watertight and biodegradable surgical patches for potential fetoscopic myelomeningocele repair. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2019;107(2):295–305.
22. Kunpalin Y, Subramaniam S, Perin S, Gerli MF, Bosteels J, Ourselin S, Deprest J, De Coppi P, David AL. Preclinical stem cell therapy in fetuses with myelomeningocele: A systematic review and meta-analysis. Prenatal diagnosis. 2021;41(3):283–300.
23. Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51(4):239–54.
24. Bergh E, Buskmiller C, Johnson A. The Future of Fetal Surgery. Obstetrics and Gynecology Clinics. 2021;48(4):745–58.
Natalie C. Campbell, 1 BA. Fourth-year Medical Student. Indiana University School of Medicine, Indianapolis, United States.
Stephen B. Trippel, 2 MD. Indiana University School of Medicine, Indianapolis, United States.
Eric A. Nauman, 3 PhD. Purdue University, West Lafayette, United States.
About the Author: Natalie Campbell is currently a 4th year medical student at Indiana University School of Medicine, Indianapolis, USA. Natalie is currently pursuing a scholarly concentration in biomedical engineering and has been inducted into Indiana University School of Medicine’s chapters of Alpha Omega Alpha and Gold Humanism Honor Society.
Correspondence: Natalie C. Campbell. Address: 340 West 10th Street, Fairbanks Hall, Suite 6200, Indianapolis, United States. Email: natccamp@iu.edu
Editor: Francisco J. Bonilla-Escobar; Student Editors: Nikoleta Tellios & Purva Shah; Copyeditor: Sebastian Diebel; Proofreader: Michael Tavolieri; Layout Editor: Fatma Monib; Process: Peer-reviewed
Cite as Campbell NC, Trippel SB, Nauman EA. Biomedical Engineering Advancements after Management of Myelomeningocele Study (MOMS): A Narrative Review. Int J Med Stud. 2022 Oct-Dec;10(3):405-12.
Copyright © 2022 Natalie C. Campbell, Stephen B. Trippel, Eric A. Nauman
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 4, April 2022