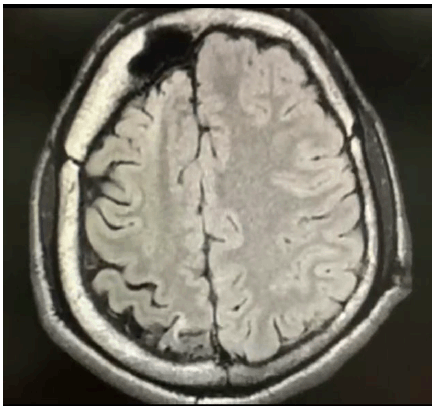
MRI Showing Right Cerebral Atrophy with Gliotic and Encephalomalacic Changes Along with Compensatory Thickening of Cranial Vault.
Highlights:Gaurav M. Urs1, Hitesh R. Doddabele2
doi: http://dx.doi.org/10.5195/ijms.2022.1217
Volume 10, Number 1: 82-85
Received 05 04 2020: Rev-request 08 05 2020: Rev-recd 02 04 2020: Accepted 25 08 2020
ABSTRACT
Background:Dyke-Davidoff-Masson syndrome (DDMS) is a neurological syndrome characterized by the presence of convulsions, facial asymmetry due to palsy of the facial nerve (CN VII), contralateral hemiplegia, and reduced intellectual capacity.
The Case:We report a case of DDMS in a 20-year-old male who is a previously known case of generalized epilepsy on medication presenting with status epilepticus and initially managed by anticonvulsants. On admission, the seizures manifested again which required the patient to be sedated with injectable anesthetics and intubated. Clinical examination showed no focal neurological deficits or neurocutaneous markers. Imaging studies showed characteristic features of DDMS which were hemiatrophy of the right cerebrum with calvarial thickening, and sinuses showing hyperpneumatization on the same side as hemiatrophy. Previous history of such episodes had been recorded and the patient was kept on strict pharmacotherapy. Failure of adherence to these led to the current presentation. The diagnosis of DDMS was kept and the patient was treated conservatively with anticonvulsants and referred to a higher center for further management.
Conclusion:DDMS, being a rare but important cause of refractory epilepsy, is easily missed on initial assessment and failure of adequate management leads to higher rates of morbidity and mortality associated with this syndrome. In cases with an atypical presentation, such as this one, a good background in radio-imaging and knowledge of the physical manifestations are required for final diagnosis.
Keywords: Seizures; Neuroimaging; Anticonvulsants; Cerebral atrophy; Hemiplegia; Dyke-Davidoff-Masson syndrome.
In the year 1993 three researchers Dyke, Davidoff, and Masson came across peculiar radiographic images of cerebral hemiatrophy and compensatory hypertrophy of calvarium and frontal sinuses in nine patients who clinically presented with seizures, facial hemiparesis, and learning/developmental disabilities - thus forming the typical presentation of this syndrome and named it as Dyke-Davidoff-Masson Syndrome (DDMS).1 This condition usually results from a perinatal insult, which further leads to the loss of neurons compromising the development of the brain either focally, or as a whole, leading to the spectrum of clinical features.2 The major concern is the occurrence of such convulsive episodes for which pharmacotherapy alone is insufficient in most of the cases, and where surgical management is eventually advised.3 We are hereby describing the clinical and radiological features of this syndrome in a young adult presenting to us with refractory seizures.
A 20-year-old male patient presented to our emergency department with sudden onset of involuntary movements of both limbs, upward gazing of eyes, frothing of the mouth, involuntary micturition, and tongue bite. The patient's attendants gave a history of 10-12 seizures since the previous night before arrival to the hospital with episodes of loss of consciousness for more than 30 minutes and post-ictal confusion for a period of 45 minutes. His seizure was managed with a dose of Lorazepam (2mg) followed by Levetiracetam (1g) intravenously. Blood samples were collected and sent for blood sugar levels, complete metabolic panel, and complete hemogram, in order to rule out the common causes of seizures. After stabilization with lorazepam and levetiracetam, the patient was in a state of post-ictal confusion, and admission to the medical intensive care unit was taken up for monitoring and further investigations. Further tests for liver and renal functions were conducted.
The patient was a known case of seizure disorders since the age of 17, with left focal onset seizures in his upper limb generalizing to both upper and lower limbs and was on pharmacotherapy (Sodium valproate 300mg BD, Phenobarbital 60mg BD, Clobazam 10mg BD). The seizure episodes started at the age of 3 and were managed under the above-mentioned antiepileptics. Seizures were usually preceded by neck pain, nausea, and involuntary movements of the right hand, diagnosed as idiopathic generalized epilepsy by the local physician, and kept as the diagnosis without further investigations or referral to a higher center. They also gave a history of episodic seizures which were managed by increasing the dosage of Clobazam to 20mg BD instead of regular dosing of 10mg BD. Consanguinity was not seen in the family tree. Uneventful perinatal history was given by the patient's attenders. There were no similar complaints in the immediate family. The parents noted learning difficulties and took him off from schooling in his first grade. He can speak in his mother tongue fluently. Motor developmental milestones were developed at appropriate ages.
On admission to the medical intensive care unit, the patient remained stable shortly for an hour and then presented with the second episode of seizures, initially with focal seizures of the left hand with secondary generalization. Patient was treated with Lorazepam 2mg, Levetiracetam 1g, Sodium Valproate 1g, Phenobarbitone 1g, following sedation with Midazolam infusion at 0.2mg/kg/hr, and mechanical ventilation, due to the seizure not being controlled by the above medications. Mechanical ventilation was continued for the next 4 days, then weaned off and extubated. On extubation, the patient remained stable and vital signs were near normal with no new onset of seizure episodes. Initially sent blood tests showed no significant findings.
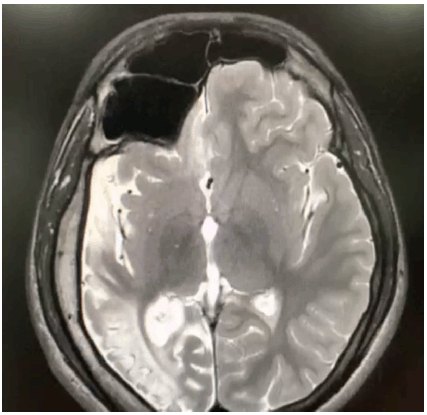
The clinical examination of the central nervous system was normal and did not reveal any neurocutaneous lesions. He scored poorly on the Mini-Mental Status Examination (14/30), with brisk tendon reflexes and flexor plantar response. A magnetic resonance imaging (MRI) of the brain was subsequently done, which revealed right cerebral atrophy, with gliotic and encephalomalacic changes together with compensatory thickening of the cranial vault (Figure 1), and enlargement of frontal and hemisphenoid sinuses on the right side, with an elevation of the right petrous edge (Figure 2). An electroencephalogram (EEG) report, which was performed 3 years ago, showed abnormal EEG changes with generalized seizure discharges and diffuse background slowing.
Figure 1.MRI Showing Right Cerebral Atrophy with Gliotic and Encephalomalacic Changes Along with Compensatory Thickening of Cranial Vault.

MRI Showing Enlargement of Frontal and Hemisphenoid Sinuses on the Right Side with Elevation of the Right Petrous Edge.
We accordingly kept a diagnosis of DDMS, managed him conservatively with the above mentioned antiepileptics, and referred him to a comprehensive center for further management upon the patient's attendees' request. The patient's attendees refused surgical intervention due to financial constraints, and are continuing the anticonvulsants. Informed consent for publication was obtained from the patient's representative.
DDMS, which is a rare but important condition commonly associated with refractory seizures, was first documented by Dyke, Davidoff, and Masson in 1933 when they noted radiographic images in a series of 9 patients with similar presentations.1 Total and subtotal cortical hemiatrophy is the pathognomonic radiological finding in this syndrome, while sometimes unilateral cerebral atrophy is also noted in the cerebral peduncles, thalamus, pons, cerebellar crossings, and surrounding areas. Neuroimaging shows prominent sulcus over the cerebrum, lateral ventricles dilated in certain parts, increase in the CSF spaces, calvarial thickening, osseous hypertrophy on the same side as the hemiatrophy with hyperpneumatization of the frontal and mastoid sinuses, and an elevated calvarium on the temporal side. Both sexes are equally affected in this case and any part of the brain can be equally involved as well, although left-sided involvement and male preponderance have been more frequently observed in one particular case study.4 The clinical features of this syndrome are hemiparesis on the same side as hemiatrophy, with an upper motor neuron type palsy of the facial nerve (CN VIII), focal or generalized convulsions, and poor intellect with a delay in the achievement of milestones either occurring alone or in combination based on the side of hemiatrophy.5
Refractory epilepsy has many etiologies. These are commonly associated with failure of adherence to antiepileptic drugs, and include seizures that are non-epileptic, misdiagnosed, or inappropriate use of medications such as inadequate dosing, drug-to-drug interactions, and lifestyle choices such as alcohol & drug abuse, stress, and sleep deprivation.6 Identification of the causative etiology is essential in planning its management, since refractory seizures are associated with high rates of morbidity and mortality. Out of the variety of tests available to investigate epilepsy, neuroimaging is the main tool used in its investigation. We came across this rare case of Dyke-Davidoff-Masson syndrome presenting as refractory seizures alone without the other typical features mentioned above.
Of the two types of cerebral hemiatrophy, the infantile subtype results from perinatal vascular insult usually involving the middle or anterior cerebral artery, coarctation of aortic arch; or common early neonatal sepsis thus presenting with the symptoms subsequently in the age group when the insult had occurred. Other, acquired, subtype of DDMS usually results from hypoxic-ischemic encephalopathy, pyrexic seizures of prolonged duration, traumatic insult, or from neoplastic or infectious etiology, along with hemorrhagic and ischemic causes.7-8 The classical MRI changes of this disease, which are hemiatrophy and hyperpneumatization of sinuses, are observed radiographically only if the causative factor has acted upon the developing brain before the age of three.9
The differential diagnosis of this presentation seen in our case includes Sturge-Weber syndrome and Rasmussen encephalitis. Also, certain syndromes like Fishman syndrome, Silver-Russell syndrome, and linear nevus syndrome have to be kept in the picture as rare but possible causes. These syndromes are recognized through neuroimaging and clinical correlation.10-11 Sturge-Weber syndrome is presented clinically by port-wine nevus on the face, epilepsy, ophthalmic manifestations primarily being increased intraocular pressure, learning difficulties, and stroke-like features occurring frequently. The underlying pathology is due to intracranial vascular anomaly and leptomeningeal angiomatosis and stasis causing the pathognomonic intracranial tram track calcification with laminar cortical necrosis leading to atrophy.12 Rasmussen encephalitis, an immune-mediated progressive chronic condition occurring commonly in the younger age group of six to eight years, with the child presenting with intractable focal onset epilepsy and cognitive defects with imaging findings similar to that of hemi cerebral atrophy but no significant calvarial changes.13 Silver-Russell syndrome is characterized by its unique facial phenotype, poor attainment of physical parameters such as height and bone length, clinodactyly, cerebral hemihypertrophy without affecting the head circumference, and no deranged mental capacity.14 Fishman syndrome is a neurocutaneous syndrome occurring rarely which presents with unilateral cranial lipomatosis, ophthalmic lipodermoid, along with seizures characterized by radiological features of cortical calcification and hemiatrophy.15 The hallmarks of linear nevus syndrome are typically facial nevus, recurrent refractory seizures, growth retardation with mental retardation, and unilateral ventricular dilatation resembling cerebral hemiatrophy.16
With the clinical features of cerebral hemiatrophy along with supportive radiological evidence of cerebral hemiatrophy, osseous hypertrophy of the skull, and compensatory hyper-pneumatization of the sinuses, DDMS has to be considered as the cause.17-18 Even though our patient had just refractory seizures and learning difficulties as the clinical features, radiographic assistance is the one that aided in the prompt diagnosis of this syndrome. Commonly affecting the pediatric population, this case is of importance since our patient is in his early adulthood.19 On further examination, patient was seen to have missed the dosing of the antiepileptics leading to the onset of the above scenario, thus being the causative etiology.
Conservative management of DDMS includes rational use of antiepileptic drugs, usually in combination since they do not easily adhere to monotherapy. If seizures are refractory, cerebral hemispherectomy is the available neurosurgical option which ensures the patient is seizure-free in about 85% of the operated cases.3 Long-term management also includes adjunctive usage of physiotherapy, occupational and speech therapy. At present, management of epilepsy is still limited to monotherapy or adjunct usage of antiseizure drugs as the first-line management. Prompt diagnosis and early adherence to antiepileptics as the medical management of the seizures along with rehabilitation of both neurological and physical activities are also essential.20
DDMS usually presents in early childhood or adolescents as refractory seizures requiring lifelong pharmacotherapy with anticonvulsants. Due to its rarity of occurrence, it is commonly missed on initial assessment. The relatively high cost of anticonvulsants, upon the background of low socioeconomic status, personal expenses for treatment, facilitates poor adherence to the drugs and thus broadens the treatment gap.21 Further studies are necessary to identify the natural course of DDMS, especially in the adult population leading to appropriate and economical management.
We are grateful to Dr. Shashikantha Bhat (Head of Department, Internal Medicine, Adichunchanagiri Hospital and Research Centre, Karnataka, India) for his guidance and support in writing the manuscript. The authors also wish to thank Dr. Shivadarshan M. (Consultant Radiologist, Adichunchanagiri Hospital and Research Centre, Karnataka, India) for his input on the images. Also, we would like to thank Dr. Tejaswi HL (Associate professor, Department of Anatomy, Adichunchanagiri Institute of Medical Sciences, Karnataka, India) for his guidance on this case report.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: GU; Data Curation: GU, HD; Resources: HD; Visualization: GU; Writing – Original Draft Preparation: GU; Writing – Review & Editing: GU, HD.
1. Dyke CG, Davidoff LM, Masson LB. Cerebral hemiatrophy with homolateral hypertrophy of the skull and sinus. Surg Gynecol Obstet. 1933;57:588-600.
2. Shetty DS, Lakhkar BN, John JR. Dyke-Davidoff-Masson syndrome. Neurol India. 2003 Mar;51(1):136.
3. Roy U, Panwar A, Mukherjee A, Biswas D. Adult Presentation of Dyke-Davidoff-Masson Syndrome: A Case Report. Case Rep Neurol. 2016 Jan 16;8(1):20-6.
4. Unal O, Tombul T, Cirak B, Anlar O, Incesu L, Kayan M. Left hemisphere and male sex dominance of cerebral hemiatrophy (Dyke-Davidoff-Masson Syndrome). Clin Imaging. 2004 May–Jun;28(3):163-5.
5. Afifi AK, Godersky JC, Menezes A, Smoker WR, Bell WE, Jacoby CG. Cerebral hemiatrophy, hypoplasia of internal carotid artery, and intracranial aneurysm. A rare association occurring in an infant. Arch Neurol. 1987 Feb;44(2):232-5.
6. Angel J Jr. Approaches to refractory epilepsy. Ann Indian Acad Neurol. 2014 Mar;17(Suppl 1):S12-7.
7. Sener RN, Jinkins JR. MR of craniocerebral hemiatrophy. Clin Imaging. 1992 Apr–Jun; 16:93-97.
8. Stred SE, Byrum CJ, Bove EL, Oliphant M. Coarctation of the midaortic arch presenting with monoparesis. Ann Thorac Surg. 1986 Aug;42(2):210-2.
9. Solomon GE, Hilal SK, Gold AP, Carter S. Natural history of acute hemiplegia of childhood. Brain. 1970 Jan 1;93(1):107-20.
10. Rao KC: Degenerative diseases and hydrocephalus; in Lee SH, Rao KC, Zimmerman RA (eds): Cranial MRI and CT. New York, McGraw-Hill, 1999, pp 212-214.
11. Zilkha A: CT of cerebral hemiatrophy. Am J Roentgenol 1980 Aug;135:259-262.
12. Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-Weber syndrome: a review. Pediatr Neurol. 2004 May;30(5):303-10.
13. Sheybani L, Schaller K, Seeck M: Rasmussen encephalitis: an update. Schweiz Arch Neurol Psychiatr 2011 Sep 7;162:225-31.
14. Qiu BP, Shi CH: Silver-Russel syndrome: a case report. World J Pediatr 2007;3:68-70.
15. Amor DJ, Kornberg AJ, Smith LJ. Encephalocraniocutaneous lipomatosis (Fishman syndrome): a rare neurocutaneous syndrome. J Paediatr Child Health. 2000 Dec;36(6):603-5.
16. Jacoby CG, Go RT, Hahn FJ. Computed tomography in cerebral hemiatrophy. AJR Am J Roentgenol. 1977 Jul;129(1):5-9.
17. Ono K, Komai K, Ikeda T. Dyke-Davidoff-Masson syndrome manifested by seizure in late childhood: a case report. J Clin Neurosci. 2003 May;10(3):367-71.
18. Aguiar PH, Liu CW, Leitão H, Issa F, Lepski G, Figueiredo EG, et.al. MR and CT imaging in the Dyke-Davidoff-Masson syndrome. Report of three cases and contribution to pathogenesis and differential diagnosis. Arq Neuropsiquiatr. 1998 Dec;56(4):803-7.
19. Abdul Rashid AM, Md Noh MSF. Dyke-Davidoff-Masson syndrome: a case report. BMC Neurol. 2018 May 29;18(1):76.
20. George P, Shenoy BR. Dyke-Davidoff-Masson Syndrome: an uncommon cause of refractory epilepsy identified by neuroimaging. Journal of Clinical and Diagnostic Research 2011;5:833-4.
21. Narain NP, Kumar R, Narain B. Dyke-Davidoff-Masson syndrome. Indian Pediatr. 2008 Nov;45(11):927-8.
Gaurav M. Urs, 1 Intern Doctor, Department of Internal Medicine, Adichunchanagiri Hospital and Research Centre, Nagamangala, Karnataka, India.
Hitesh R. Doddabele, 2 Third-year medical student, Department of Internal Medicine, Adichunchanagiri Hospital and Research Centre, Nagamangala, Karnataka, India.
About the Author: Gaurav M Urs recently graduated from Adichunchanagiri Institute of Medical Sciences, Karnataka, India, and is currently working at Adichunchanagiri Hospital and Research Centre, Karnataka, India as an Intern Doctor. Hitesh R Doddabele is a third-year medical student at Adichunchanagiri Institute of Medical Sciences, Karnataka, India.
Correspondence: Gaurav M. Urs. Address: NH75, Karnataka 571448, India. Email: gauravmurs@bgsaims.edu.in
Editor: Francisco J. Bonilla-Escobar. Student Editors: Ciara Egan. Copyeditor: Adnan Mujanovic. Proofreader: Madeleine J. Cox. Layout Editor: Judie Joo. Process: Peer-reviewed.
Cite as: Urs GM, R. Doddabele H. Dyke-Davidoff-Masson Syndrome: A Case Report. Int J Med Stud. 2022 Jan-Mar;10(1):82-85.
Copyright © 2022 Gaurav M. Urs, Hitesh R. Doddabele
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 1, Jan-Mar 2020