Review
Effects of COVID-19 on Multiple Sclerosis Relapse: A Comprehensive Review
Matthew Topolski1, Varun Soti2
doi: http://dx.doi.org/10.5195/ijms.2022.1241
Volume 10, Number 2: 192-201
Received 07 10 2021:
Rev-request 06 12 2021:
Rev-request 03 11 2021:
Rev-request 28 01 2022:
Rev-recd 12 11 2021:
Rev-recd 30 12 2021:
Rev-recd 09 02 2022:
Accepted 13 02 2022
ABSTRACT
Multiple Sclerosis is a chronic inflammatory disease. It is characterized by demyelinating
lesions throughout the central nervous system. Patients with multiple sclerosis are
a vulnerable population to coronavirus disease-2019 (COVID-19). This review focuses
on the effects of COVID-19 on relapse and symptom exacerbation in multiple sclerosis
patients and their treatment. It highlights how the blood-brain barrier may be compromised
by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), allowing inflammatory
mediators and lymphocytes to infiltrate the central nervous system. This may increase
the risk of relapse in multiple sclerosis patients. Also, in patients with no prior
history of multiple sclerosis, COVID-19 has been found to impact multiple sclerosis
onset and pathogenesis. However, more comprehensive research is required to fully
understand the interplay between multiple sclerosis and COVID-19.
Keywords:
Multiple Sclerosis;
Coronavirus Disease-2019;
COVID-19;
SARS-CoV-2;
Disease Exacerbation;
Blood-Brain Barrier;
Neurologic Symptoms (Source: MeSH-NLM).
Introduction
The coronavirus disease 2019 (COVID-19) first emerged in Wuhan, China, in December
2019.1 However, COVID-19 rapidly spread across the globe over the next six months and has
affected every aspect of healthcare.2 COVID-19 results from infection by severe acute respiratory syndrome coronavirus
2 (SARS-CoV-2). Although the infection has its main site of pathophysiologic significance
at the pulmonary level, a number of multiple sequelae, signs and symptoms, and associated
pathologies have been observable in multiple body systems, including the nervous system;
there has been a growing number of neurologic problems associated with the SARS-CoV-2
infection, including complications with multiple sclerosis (MS).3–5
MS is a chronic inflammatory disease of the central nervous system (CNS) characterized
by demyelinating lesions that can lead to various neurologic dysfunction, including
cognitive dysfunction, dysesthesia, hyperreflexia, hypoesthesia, paresthesia, and
visual deficits (diplopia, nystagmus, and optic neuritis), depending on the location
and severity of inflammatory lesions.6
The most common disease course in MS is relapsing-remitting multiple sclerosis (RRMS);
it is characterized by acute exacerbations of symptoms, followed by more extended
periods of remission. These short exacerbations are also called relapses and consist
of days to weeks of fully or partially reversible neurological disability. Principal
manifestations of relapses are monocular visual loss, limb weakness and, or sensory
loss, double vision, and ataxia.6
The exact causes of relapses remain unknown, but relapse rates have been correlated
with times of increased stress.7 Other disease courses of MS involve clinically isolated syndrome (CIS), primary progressive
multiple sclerosis (PPMS), and secondary progressive multiple sclerosis (SPMS). The
CIS is diagnosed after the first episode of a demyelinating attack. It presents as
a neurologic deficit for more than 24 hours. PPMS is a progressive form in which neurologic
deficits accumulate in the absence of relapse and do not regress to baseline despite
treatment; whereas SPMS often occurs as a later stage of RRMS, where neurologic deficits
do not return to baseline after relapses, and deficits accumulate after each relapse.8
This review primarily focuses on the RRMS, the most common course characterized by
relapses. MS relapse and even its onset have been known to be impacted by viral infections.7, 9 The stress of a viral infection combined with the host immune response creates a
proinflammatory environment and increases the risk of relapse in Persons with Multiple
Sclerosis (PwMS). However, the literature is lacking regarding SARS-CoV-2 and its
potential impact on the onset and relapse in PwMS. Therefore, this review highlights
the neurological effects of COVID-19 on PwMS and its impact on their disease status
and symptom exacerbation.
Methods
Strategies for Literature Search and Study Selection
We conducted a literature search through the PubMed and EBSCO databases from March
2020 through July 2021 for studies measuring relapses in PwMS who had been infected
by COVID-19. Inclusion criteria included: 1) studies being written in English; 2)
any case report, retrospective cohort study, and prospective cohort study that included
PwMS who were infected with SARS-CoV-2; 3) studies that measured neurologic symptom
exacerbation or relapse. We used the following search terms: “Coronavirus Multiple
Sclerosis,” “Coronavirus MS Relapse,” “Coronavirus MS Exacerbation,” “COVID-19 Multiple
Sclerosis,” “COVID-19 MS Relapse,” “COVID-19 MS Exacerbation,” “SARS-CoV-2 Multiple
Sclerosis,” “SARS-CoV-2 MS Relapse,” “SARS-CoV-2 MS Exacerbation.”
Our search resulted in 399 articles in total. Of those, one study was not written
in English, 390 were not case reports, retrospective cohort studies, or prospective
cohort studies that included PwMS infected with SARS-CoV-2, and one did not measure
neurologic symptom exacerbation or relapse. Of the seven studies meeting the inclusion
criteria, two were retrospective studies, one was a prospective cohort study, one
was an observational study, and three were case reports. The level of evidence for
the included studies was determined based on the previous literature.10 The methodology used in the review is illustrated in Figure 1, which is based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines.11
Figure 1
Method Employed to Search Literature.
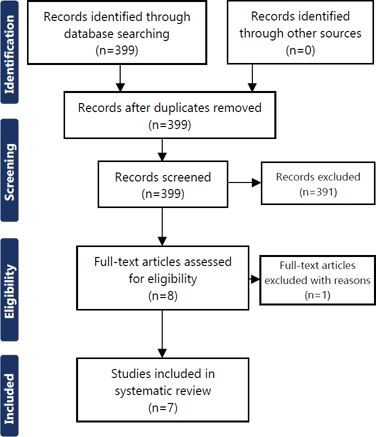
Legend: We searched PubMed and EBSCO databases from March 2020 to July 2021. The search terms
“Coronavirus Multiple Sclerosis,” “Coronavirus MS Relapse,” “Coronavirus MS Exacerbation,”
“COVID-19 Multiple Sclerosis,” “COVID-19 MS Relapse,” “COVID-19 MS Exacerbation,”
“SARS-CoV-2 Multiple Sclerosis,” “SARS-CoV-2 MS Relapse,” “SARS-CoV-2 MS Exacerbation”
were utilized. This yielded 399 articles, of which 7 studies meeting the inclusion
criteria for this review paper were selected. COVID-19, coronavirus disease 2019;
MS, multiple sclerosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus
2.
Results
Pathophysiology
MS is an autoimmune disease characterized by plaque-like sclerosis found throughout
the CNS. Its most common disease course is RRMS, which is identified by symptom exacerbations;
during exacerbations, acute demyelinating attacks occur between more prolonged periods
of quiescence.8 Throughout these demyelinating episodes, myelin basic protein (MBP), a critical component
of the myelin sheath, is adversely impacted.12 These inflammatory lesions are more significantly found in the white matter but have
also been seen in the gray matter; lesions are widely observed in the periventricular
region, juxtacortical areas, infratentorial region, and spinal cord.13
The MS diagnosis relies on the dissemination of the disease in space and time as defined
by the revised 2017 McDonald criteria.14 Typical onset of the disease occurs between the ages of 20 and 40 years old; inflammatory
lesions are thought to result from pro-inflammatory factors and demyelination that
occurs in the CNS after the blood-brain barrier (BBB) has been compromised.15
Although the exact mechanism of the autoimmune action against CNS antigens in MS remains
undetermined, the bulk of evidence attributes pathology to both the adaptive and innate
immune responses in an attack against myelin and oligodendrocytes. Both clusters of
differentiation (CD) 4+ and CD8+ T cells have been found in MS lesions, suggesting
cell-mediated immunity in the inflammatory lesions.16 T cells are the major driving factor of experimental autoimmune encephalitis in a
murine MS model. The success of therapies that limit T cell access to the CNS also
supports the role of cell-mediated immunity in MS pathology.17 Moreover, the recent success of B cell-depleting therapies in MS treatment has also
suggested a more prominent role of the humoral response in MS pathology.18 Furthermore, B cells have been shown to activate autoreactive T cells that target
the brain.19
Macrophages of the innate immune system promote the inflammatory response of T and
B cells and execute the tissue damage seen in MS.6, 20 Microglial cells of the CNS also contribute to pathology through secretion of the
inflammatory cytokines, chemokines, and free radicals.21 The autoimmune mechanism of MS pathogenesis is illustrated in Figure 2.
Figure 2
Autoimmune Mechanism of Multiple Sclerosis.
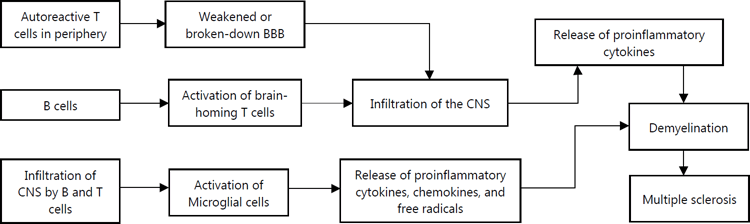
Legend: Autoreactive T cells in the periphery infiltrate the CNS through a weakened or broken-down
BBB, releasing inflammatory cytokines, attacking myelin and oligodendrocytes, and
causing demyelination. B cells activate brain-homing T cells in the periphery, further
breaking through the CNS and resulting in demyelination through a similar mechanism.
Microglial cells are activated by the infiltration of T and B cells in the CNS, releasing
more proinflammatory cytokines, chemokines, and free radicals, contributing to demyelination.
BBB, blood-brain barrier; CNS, central nervous system.
Effect of Viral Infections in Multiple Sclerosis
Viral infections, mostly considered as the environmental factor, have been known to
induce relapses in PwMS. Significantly, upper respiratory infections (URIs) have long
been correlated with MS relapse risk.22, 23 The extensive history of viral infection and MS outcomes have been seen in members
of the Herpesviridae family, including Epstein-Barr virus, Varicella-Zoster virus,
and human herpesvirus 6.24–27 Also, parainfluenzas, adenoviruses, and coronaviruses have been correlated with the
risk of MS relapse.23, 28 Furthermore, multiple viral infections have been shown to increase the risk for relapse,
suggesting a common mechanism across the viral immune response. This could be from
increased permeability of the BBB due to antiviral cytokines or molecular mimicry
of viral and host proteins.29
Coronaviruses have been previously reported to be involved and complicate MS pathophysiological
processes. A postmortem study found human coronavirus (HCV) 229E ribonucleic acid
(RNA) in CNS tissues of 4 out of 11 MS patients compared to control groups (6 neurological
controls and 5 healthy controls). The specific specimens were scraped from white matter
plaques, typical gray and white matter, and tissues from the cervical cord.30 Four of the neurological controls had Alzheimer's disease, one had ischemic vascular
disease, and one had subacute meningoencephalitis. Another research group has corroborated
the presence of coronavirus RNA in CNS tissues of PwMS. During the autopsy, researchers
found that 11 out of 21 MS patients had HCV RNA in their CNS tissue obtained from
the cerebral cortex, brainstem, and spinal cord compared to the control group.31
Based on these histopathological findings, it can be inferred that HCV compromised
the structural integrity of the BBB and invaded the specific CNS areas containing
MS lesions, and caused pathophysiological complications in already vulnerable MS patients.30,31
Interestingly, not only have coronaviruses been reported to have harmful effects on
MS pathophysiology, but also, they have been shown to indirectly promote demyelination
through T cell activation in cell lines obtained from MS patients.32 A study found that 29% of T cell lines from MS patients showed MBP and HCV 229E cross-reactivity
compared to only 1.3% of T cell lines from healthy controls. Furthermore, 4 out of
16 MS patients displayed reciprocal cross-reactivity profiles while none of the healthy
controls did.32 These findings further indicate the possible environmental trigger of coronaviruses
on MS pathogenesis and pathophysiology.30–32 Thus, the SARS-CoV-2 strains are likely to have similar effects to previously studied
coronaviruses and other viral infections on MS status.
COVID-19 and its Neurological Manifestations
SARS-CoV-2, which causes COVID-19, has a well-described cell entry mechanism.33 Antigen presentation by antigen-presenting cells (APCs) is crucial to antiviral cell-mediated
immunity. A recent study suggests a defect in the MHC class II gene expression for
the presentation of SARS-CoV-2 by APCs.34
The polymorphic nature of the MHC region of the human genome plays an essential role
in individual susceptibility to diseases such as MS.35 The innate and adaptive immune system response to coronaviruses is integral to the
infection's clinical presentation; the innate immune response is triggered by pattern
recognition receptors (PRRs), recognition by PRRs triggers a downstream signaling
cascade that results in the secretion of inflammatory cytokines such as interferons
(IFN), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, and IL-6.35 Humoral immunity to SARS-CoV-2 can be seen through the presence of antibodies directed
to the viral surface glycoproteins S protein and N protein of the SARS-CoV-2. APCs
trigger the cell-mediated immune response by presenting antigens to virus-specific
CD4+ and CD8+ T cell antigen receptors.36
Upon activation of the innate and adaptive immune systems by SARS-CoV-2, another massive
quantity of proinflammatory cytokines and chemokines are produced from immune effector
cells; this immune-mediated cytokine storm has been attributed to the severe clinical
presentation of acute respiratory distress syndrome in COVID-19 patients.35, 36 Thus, this cytokine storm could lead to increased permeability through cytokine-mediated
inflammation at the BBB. This could be detrimental to more susceptible patients with
neurodegenerative conditions, for instance, MS patients.
Beyond the significant respiratory complaints of COVID-19, there has been an increasing
number of reported neurological complications of the disease.37–40 A nationwide retrospective observational study in Italy showed that 72.1% of the
646 patients surveyed reported neurological symptoms during their COVID-19 infection.
Headache was the most reported symptom (41.1%), followed by smell (37.9%) and taste
(36.8%) impairment.5 A significant number of people have been reported to develop psychiatric issues,
including depression, anxiety, and stress, particularly those with pre-existing mental
conditions.41–43 Moreover, there have also been reports of more serious neurological complications
of COVID-19, such as Guillain Barre syndrome and acute transverse myelitis.44–46 In addition, as mentioned before, some studies have shown the correlation between
coronaviruses and demyelination.47–49
There have been several proposed mechanisms of coronavirus infection of the nervous
system. Viruses have been shown to migrate through retrograde or anterograde neuronal
axonal transport.37, 50 This has also been seen in the olfactory and trigeminal nerves, leading to CNS infection
in mouse models.51 The binding of SARS-CoV-2 to angiotensin-converting enzyme 2 receptors on vascular
endothelium may damage the BBB, leading to its entry into the CNS,52 thus allowing infiltration of the activated immune response into the CNS. The suggested
breakdown of BBB by SARS-CoV-2 may shed light on the pathophysiologic mechanism of
how MS patients are significantly impacted by COVID-19. Also, PwMS have been considered
particularly vulnerable to SARS-CoV-2 infection due to high disability rates and increased
susceptibility to infection.53
MS Relapse and COVID-19
In an observational study of MS patients with COVID-19 (72 MS patients), 21.1% reported
neurologic symptoms suggestive of relapse.54 A retrospective cohort study by Etemadifar et al. found 7.14% of the 56 PwMS experienced
a relapse from the period of two weeks before and six months after recovering from
COVID-19.55
Another retrospective study assessing 41 PwMS found an increased relapse rate of 0.017
attacks per “at-risk” week compared to 0.007 attacks per week during a not “at-risk”
period of the two years prior. The “at-risk” period was defined as the two weeks before
and five weeks after COVID-19 infection.56 A more extensive study performed in the United Kingdom found 57% of PwMS (230/404)
experienced MS exacerbation during the time of their COVID-19 infection.57 The key findings of some studies about MS relapse in PwMS infected with SARS-CoV-2
are summarized in Table 1.
Table 1.
Multiple Sclerosis Relapse in COVID-19 Patients.
| Studies |
Level of Evidence |
Patients |
Study Findings |
Study Bias |
p-value |
| Barzegar et al. (2021) – Retrospective cohort study |
3 |
41 RRMS with COVID-19 |
- Five patients (12.2%) displayed neurological symptoms consistent with relapse during
the at-risk period of SARS-CoV-2 infection.
- The study demonstrated increased risk of relapse of these patients during their at-risk
period compared to the previous 2 years during the not at-risk period.
|
- Study did not compare results of SARS-CoV-2-infected PwMS to non-infected PwMS.
- Instead, this study compared the at-risk period (2 weeks before through 5 weeks after
infection) to the not at-risk period (previous 2 years).
|
0.034 |
| Etemadifar et al. (2021) – Retrospective cohort study |
3 |
125 RRMS patients (56 with COVID-19 and 69 without COVID-19) |
- Study reported a lower incidence rate of neurological symptom exacerbation in the
PwMS with COVID-19 (7.14%) in the six months following confirmed infection with SARS-CoV-2
compared to PwMS without COVID-19 in the six months measured from Jun 1, 2020 – November
1, 2020 (26.09%).
|
- Participants were contacted biweekly through telephone surveys. This likely increased
the likelihood of exaggerated reporting of symptoms.
|
0.006 |
| Fragoso et al. (2021) – Case report |
4 |
1 PwMS |
- Study of a healthy individual who was diagnosed with MS six months after having COVID-19.
- The temporal relationship of the COVID-19 onset and MS diagnosis are thought to be
related.
|
- Six months post SARS-CoV-2 infection is a substantial time to develop MS independent
of any viral infection let alone SARS-CoV-2.
- Many other factors could have played a role in disease onset in that time.
|
Not applicable |
| Garjani et al. (2021) – Prospective cohort |
3 |
404 PwMS (277 RRMS, 65 SPMS, 39 PPMS, 23 Non-defined MS) |
- Study showed 230/404 PwMS (56.9%) and COVID-19 reported symptom exacerbation during
or soon after infection with SARS-CoV-2 from July 20, 2020, through January 25, 2021.
|
- Study did not have a control group of PwMS who were not infected with SARS-CoV-2.
- Use of an online questionnaire to assess symptom exacerbation could have led to increased
responses of symptom exacerbation.
- The study's protocol did not require PwMS with confirmed SARS-CoV-2 diagnosis. Patients
who had symptoms consistent with COVID-19 were included in the study.
- Study included patients with SPMS, PPMS, and non-defined types of MS rather than just
RRMS patients.
|
No statistically significant difference between PwMS with COVID-19 who reported MS
symptom exacerbation versus PwMS with COVID-19 who did not report MS symptom exacerbation.
|
| Moore et al. (2021) – Case report |
4 |
1 PwMS |
- Patient presented with concurrent MS onset and SARS-CoV-2 infection.
|
- Patient presented in case had glaucoma and underwent prior laser ablation treatment.
This could have impacted the retinal ganglionic cells and triggering structural changes
in the blood-brain barrier, most likely predisposing him to developing MS. This was
not adequately addressed by the authors.
|
Not applicable. |
| Palao et al. (2020) – Case report |
4 |
1 PwMS |
- Patient presented with signs of MS onset (visual acuity deficits and periventricular
lesions on the MRI). She had symptoms of COVID-19 (anosmia and ageusia) 2-3 weeks
prior to presentation. Serological testing revealed immunoglobulin M and G antibodies
to SARS-CoV-2. This suggests MS onset after recent infection with SARS-CoV-2.
|
- The authors assumed the MS pathogenic process started prior COVID-19 disease.
- The SARS-CoV-2 PCR testing protocol in the cerebrospinal fluid was not properly validated.
|
Not applicable. |
| Parrota et al. (2020) – Observational study |
3 |
76 patients: 72 PwMS [55 RRMS, 17 progressive MS (SPMS, PPMS)] and 4 with related
disorders (chronic relapsing inflammatory optic neuropathy, myelin oligodendrocyte
glycoprotein-immunoglobulin G–associated disorder spectrum disorder, neurosarcoidosis,
and neuromyelitis optica)
|
- Study measured clinical outcomes in PwMS and related conditions after infection with
SARS-CoV-2.
- 21.1% of study participants reported neurological symptoms suggestive of a relapse.
|
- Patients were not randomly selected.
- Study included four participants who were not diagnosed with MS.
- Authors did not make any statistically significant comparisons between study groups.
|
Not reported. |
Legend: %, percentage; COVID-19, coronavirus disease 2019; MRI, magnetic resonance imaging;
MS, multiple sclerosis; PwMS, persons with multiple sclerosis; RRMS, relapsing remitting
multiple sclerosis; p-value, probability value; PPMS, primary progressive multiple
sclerosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPMS, secondary
progressive multiple sclerosis.
Although these studies present evidence of relapse in MS patients with COVID-19, there
is a tremendous variation in the percentage of MS patients suffering from relapse
between the studies. This might be attributed to the patient age group and MS status;
older patients, in general, have a weaker immune system, and MS geriatric patients
placed on disease-modifying therapies (DMTs) are at an even greater risk of contracting
infection, let alone SARS-CoV-2.58 Thus, had the clinical trials controlled for the age and MS status, there is a more
likelihood for more extensive and enormous evidence of MS relapse in COVID-19 PwMS.
In addition to these studies, three case reports described recent or concurrent COVID-19
infection with an initial MS event and diagnosis. A 27-year-old female presented with
MS symptoms, including dysesthesia, hyperreflexia, and hypoesthesia six months after
developing COVID-19. The patient was diagnosed with MS that was confirmed by gadolinium-enhancing
lesions on the magnetic resonance image (MRI) and the presence of oligoclonal bands
in her cerebrospinal fluid (CSF).59 The temporal relationship between MS and COVID-19 could be explained by SARS-CoV-2-induced
processes.
In another case report, a 29-year-old female with a history of asthma presented with
COVID-19 symptoms, including anosmia, dysgeusia, asthenia, and proximal myalgias in
her limbs that disappeared within a week after developing COVID-19. She presented
two weeks later with a ten-day history of right visual acuity deficits (typical MS
symptom). SARS-CoV-2 Immunoglobulin (Ig) M/IgG immunological testing was positive,
confirming past infection of the virus. Oligoclonal bands were present in CSF. MRI
displayed optic nerve lesions with contrast enhancement and sparse demyelinating lesions
in the brain, confirming MS.60 Before contracting SARS-CoV-2, the patient did not have a medical history of MS,
and within two weeks of infection, she exhibited MS symptoms and received a confirmed
MS diagnosis. Hence, there is a possibility, and unbeknownst to the investigators,
the patient might have been genetically predisposed to developing MS. And exposure
to SARS-CoV-2 would have triggered MS pathogenesis and resulted in her clinical manifestations.
Yet another case report of a 28-year-old male presented with a two-day history of
binocular diplopia was found to have MS and COVID-19 infection concurrently. The patient's
COVID-19 symptoms of sore throat, cough, anosmia, and headache had started two weeks
before diplopia,61 indicating a possible link between MS onset and SARS-CoV-2 infection. However, more
research is required to investigate and understand the relationship between MS onset/pathogenesis
and COVID-19.
The research findings are evidence of COVID-19's role in symptom exacerbation in PwMS.
Infection with SARS-CoV-2 can lead to MS onset and pathogenesis and trigger complex
pathophysiological changes, resulting in a relapse in MS patients.
However, there are several limitations to the interpretations of these studies' results
(Table 2). First, the definition of relapse or exacerbation varies between studies. A couple
of research studies used a formal definition of relapse involving the new onset of
symptoms lasting more than 24 hours. However, one study defined relapse as any neurologic
symptom that suggested a recurrence. Second, the period utilized to measure COVID-19-related
exacerbations was not consistent. One research group used a period of two weeks before
COVID-19 infection to six months after the illness, while another group only utilized
the duration patients were infected with COVID-19 as the time frame for measuring
relapse. Future studies enrolling larger cohorts with a clear definition of MS relapse
and a consistent timeframe for measuring MS relapse will be required to draw further
inferences.
Table 2.
Variations Between Research Studies on MS Patients with COVID-19.
| Studies |
Period measured for PwMS with COVID-19 |
Definition of relapse |
| Parrota et al. (2020) |
March 16, 2020 - April 30, 2020 |
Neurologic symptom recurrence suggestive of a relapse |
| Etemadifar et al. (2021) |
Two weeks before and six months after COVID symptoms |
Development of a new neurologic abnormality or worsening of a pre-existing symptom
for more than 24 hours
|
| Barzegar et al. (2021) |
Two weeks before until five weeks after COVID-19 onset |
Worsening of pre-existing symptoms or developing new symptoms, in the absence of fever,
lasting at least 24 hours, after at least 30 days of improvement and stability, confirmed
by presence of gadolinium enhancement on MRI
|
| Garjani et al. (2021) |
During or soon after COVID infection July 20, 2020 – January 25, 2021 |
Development of new MS symptoms, worsening of pre-existing MS symptoms, or experiencing
both
|
| Fragoso et al. (2021) |
Six months |
New diagnosis by McDonald criteria |
| Palao et al. (2020) |
Two weeks |
New diagnosis by McDonald criteria |
| Moore et al. (2021) |
Two weeks |
New diagnosis by McDonald criteria |
Legend: PwMS, persons with multiple sclerosis; MRI, magnetic resonance image.
Treatment of MS Patients with COVID-19
Treating COVID-19 patients with MS safely and effectively is critical partly because
MS patients are on DMTs, which can be a crucial risk factor for COVID-19. Patients
on immunomodulating therapies have been shown to have an increased risk of developing
COVID-19 but not necessarily the increased risk of severity of COVID-19.62 Despite an increased risk of COVID-19, some studies have shown better prognoses for
COVID-19 in MS patients treated with B cell-depleting therapies such as Ocrelizumab
and Rituximab measured by the severity of symptoms.63, 64 The results of these studies suggest that a suppressed immune system limits the body's
harmful response to SARS-CoV-2 infection. Another study demonstrated a decreased risk
of COVID-19 in patients being treated with IFN and glatiramer acetate.65 While these findings are optimistic, other studies have found that treatment of MS
with sphingosine-1-phosphate modulators (Fingolimod) has shown a more significant
severe disease course of COVID-19.66 The worst clinical outcomes of SARS-CoV-2 infection have been seen in PwMS who are
not on any DMTs and PwMS with comorbidities associated with worsened outcomes such
as male gender, obesity, and advanced age.67
Remdesivir
There have not been any studies regarding the treatment of COVID-19 in PwMS. Although
the treatment of COVID-19 patients depends on the individual clinical presentation,
only one drug (up to the writing of this review) has received the full United States
Food and Drug Administration (FDA) approval for the treatment of COVID-19 patients—Remdesivir.
It is a parenteral antiviral drug acting as an adenosine analog to disrupt viral RNA
production through host RNA-dependent RNA polymerase.68 However, to our knowledge, there has not been any research reporting the use, benefits,
and adverse effects of Remdesivir in COVID-19 patients with MS or other patients on
DMTs.
Immunization
The National Multiple Sclerosis Society currently recommends that most PwMS get vaccinated
for COVID-19.69 The consensus of previous inactivated vaccines in PwMS is that these vaccinations
are safe and recommended for most PwMS.70 Still, there is less known about live-attenuated vaccinations in PwMS. Vaccine safety
and efficacy in PwMS can be primarily attributed to the DMTs of the patient. With
many DMTs suppressing the immune system, a weakened vaccine response leads to decreased
immunity. Furthermore, live-attenuated vaccines can be contraindicated in patients
receiving immunosuppressive treatment due to the potential for vaccine-transmitted
disease.71, 72
Treatment with IFN-beta, Glatiramer acetate, Teriflunomide, Natalizumab, and Fumarates
have not been shown to decrease efficacy in other inactivated vaccines and are not
expected to show reduced effectiveness in the COVID-19 vaccine.73 The worst vaccine efficacies are seen in patients taking B cell-depleting therapies
such as Ocrelizumab, Rituximab, and Alemtuzumab.74–76 For patients on these therapies, the timing of vaccines and treatment is a crucial
determining factor of vaccine efficacy.71, 77
The three vaccines approved by the FDA in the United States are the BNT162b2 vaccine developed by Pfizer-BioNTech, the messenger RNA-1273 vaccine developed by Moderna, and the Ad.26.COV2.S vaccine by Janssen Biotech, Inc., a Janssen Pharmaceutical company of Johnson & Johnson.
Thus far, few studies have been conducted regarding vaccine safety and efficacy in
PwMS.
In a large observational study, 555 PwMS were vaccinated with at least one dose of
the BNT162b2 vaccine (435 received both doses). No life-threatening reactions or anaphylaxis events were
reported after either dose. Common adverse effects were injection site pain, fatigue,
headache, muscle/joint pain, and flu-like symptoms. Of the 388 RRMS patients who received
the first dose, 2.1% experienced a relapse within 10-19 days after injection. Of the
306 RRMS patients who received the second dose, 1.6% experienced a relapse within
14-21 days of injection. These rates were compared to corresponding periods of previous
years of RRMS patients who presented for acute relapses in 2017, 2018, 2019, and 2020.
The number of acute relapses divided by the number of patients in these years was
2.7%, 2.9%, 2.6%, and 2.3%, respectively.78 Thus, this study did not demonstrate any increased risk of relapse in patients who
received the Pfizer vaccine.
Conclusion
SARS-CoV-2 can increase the relapse rates in MS patients, most likely by compromising
the structural integrity of the BBB. Although, based on these study findings, it is
evident that SARS-CoV-2 can trigger MS onset and pathogenesis, more research will
be needed to further understand the underlying pathophysiologic dynamics between COVID-19
and MS. Even though COVID-19 vaccines have been safer in MS patients and have not
altered MS status, a further understanding of the relationship between COVID-19 and
MS is crucial in managing MS patients with COVID-19 on immunomodulating therapies.
Conflict of Interest Statement & Funding
The authors declare that they have no competing interests.
Author Contributions
Data curation, Formal Analysis, Investigation, Software, Visualization, Writing-Original
Draft Preparation, Writing- Review & Editing: MT; Methodology, Project Administration,
Supervision, Validation: VS; Conceptualization, Resources: MT, VS.
Acknowledgments
Learning Resource Center at Lake Erie College of Osteopathic Medicine, Elmira, NY.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
2. Prahalad A, Soti V. Coronavirus disease 2019: an overview. Int J Community Med Public Health. 2021;10(8):5094–5100.
3. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic
review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22.
5. Kacem I, Gharbi A, Harizi C, Souissi E, Safer M, Nasri A, et al. Characteristics, onset, and evolution of neurological symptoms in patients with COVID-19. Neurol Sci. 2021;42(1):39–46.
6. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180.
7. Artemiadis AK, Anagnostouli MC, Alexopoulos EC. Stress as a risk factor for multiple sclerosis onset or relapse: a systematic review. Neuroepidemiology. 2011;36(2):109–20.
8. Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391(10130):1622–36.
9. Steelman AJ. Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front Immunol. 2015;6:520.
10. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–10.
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
12. Krugmann B, Radulescu A, Appavou MS, Koutsioubas A, Stingaciu LR, Dulle M, et al. Membrane stiffness and myelin basic protein binding strength as molecular origin of
multiple sclerosis. Sci Rep. 2020;10(1):16691.
13. Filippi M, Preziosa P, Banwell BL, Barkhof F, Ciccarelli O, De Stefano N, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical
guidelines. Brain. 2019;142(7):1858–75.
14. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73.
15. Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9(6):540–9.
16. Arneth B. Activated CD4+ and CD8+ T cell proportions in multiple sclerosis patients. Inflammation. 2016;39(6):2040–4.
17. Arbour N, Prat A. Roles of CD4 and CD8 T lymphocytes in multiple sclerosis and experimental autoimmune
encephalomyelitis. In Neuroinflammation, S. David (Ed.) 2015. p. 39–52.
18. Greenfield AL, Hauser SL. B-cell therapy for multiple sclerosis: Entering an era. Ann Neurol. 2018;83(1):13–26.
19. Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, et al. Memory B cells activate brain-homing, autoreactive CD4(+) T cells in multiple sclerosis. Cell. 2018;175(1):85–100 e23.
20. van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T cells driving multiple sclerosis: Identity, mechanisms and potential triggers. Front Immunol. 2020;11:760.
21. Luo C, Jian C, Liao Y, Huang Q, Wu Y, Liu X, et al. The role of microglia in multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:1661–1667.
22. Kriesel JD, White A, Hayden FG, Spruance SL, Petajan J. Multiple sclerosis attacks are associated with picornavirus infections. Mult Scler. 2004;10(2):145–8.
23. Edwards S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with
viral upper respiratory tract infections in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64(6):736–41.
24. Levin LI, Munger KL, O'Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol. 2010;67(6):824–30.
25. Marrie RA, Wolfson C. Multiple sclerosis and varicella zoster virus infection: a review. Epidemiol Infect. 2001;127(2):315–25.
26. Kang JH, Sheu JJ, Kao S, Lin HC. Increased risk of multiple sclerosis following herpes zoster: a nationwide, population-based
study. J Infect Dis. 2011;204(2):188–92.
27. Tomsone V, Logina I, Millers A, Chapenko S, Kozireva S, Murovska M. Association of human herpesvirus 6 and human herpesvirus 7 with demyelinating diseases
of the nervous system. J Neurovirol. 2001;7(6):564–9.
28. Andersen O, Lygner PE, Bergstrom T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological
study. J Neurol. 1993;240(7):417–22.
29. Chen Z, Li G. Immune response and blood–brain barrier dysfunction during viral neuroinvasion. Innate Immunity. 2021;27(2):109–17.
30. Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191(1):502–5.
31. Murray RS, MacMillan B, Cabirac G, Burks JS. Detection of coronavirus RNA in CNS tissue of multiple sclerosis and control patients. Adv Exp Med Biol. 1990;276:505–10.
32. Talbot PJ, Paquette JS, Ciurli C, Antel JP, Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple
sclerosis. Ann Neurol. 1996;39(2):233–40.
33. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences. 2020;117(21):11727–34.
34. Saichi M, Ladjemi MZ, Korniotis S, Rousseau C, Ait Hamou Z, Massenet-Regad L, et al. Single-cell RNA sequencing of blood antigen-presenting cells in severe COVID-19 reveals
multi-process defects in antiviral immunity. Nat Cell Biol. 2021;23(5):538–51.
35. Rowaiye AB, Okpalefe OA, Onuh Adejoke O, Ogidigo JO, Hannah Oladipo O, Ogu AC, et al. Attenuating the effects of novel COVID-19 (SARS-CoV-2) infection-induced cytokine
storm and the implications. J Inflamm Res. 2021;14:1487–1510.
36. Triggle CR, Bansal D, Ding H, Islam MM, Farag E, Hadi HA, et al. A comprehensive review of viral characteristics, transmission, pathophysiology, immune
response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the
pandemic. Front Immunol. 2021;12:631139.
37. Boziki MK, Mentis AA, Shumilina M, Makshakov G, Evdoshenko E, Grigoriadis N. COVID-19 immunopathology and the central nervous system: Implication for multiple
sclerosis and other autoimmune diseases with associated demyelination. Brain Sci. 2020;10(6):345.
38. Kumar D, Jahan S, Khan A, Siddiqui AJ, Redhu NS, Wahajuddin, et al. Neurological manifestation of SARS-CoV-2 induced inflammation and possible therapeutic
strategies against COVID-19. Mol Neurobiol. 2021;58(7):3434.
39. Mahammedi A, Saba L, Vagal A, Leali M, Rossi A, Gaskill M, et al. Imaging of neurologic disease in hospitalized patients with COVID-19: An Italian multicenter
retrospective observational study. Radiology. 2020;297(2):E270–3.
40. Peterson CJ, Sarangi A, Bangash F. Neurological sequelae of COVID-19: a review. Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):122.
41. Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage
of the 2019 coronavirus disease (COVID-19) epidemic among the general population in
China. Int J Environ Res Public Health. 2020;17(5):1729.
42. Zhang SX, Wang Y, Rauch A, Wei F. Unprecedented disruption of lives and work: Health, distress and life satisfaction
of working adults in China one month into the COVID-19 outbreak. Psychiatry Res. 2020;288:112958.
43. Kandis W, Ashish S, Yasin I. The psychiatric effects of COVID-19 thus far: a review of the current literature. The Southwest Respiratory and Critical Care Chronicles. 2020;8(35).
44. Finsterer J, Scorza FA. Guillain-Barre syndrome in 220 patients with COVID-19. Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):55.
45. Fumery T, Baudar C, Ossemann M, London F. Longitudinally extensive transverse myelitis following acute COVID-19 infection. Mult Scler Relat Disord. 2021;48:102723.
46. Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13(8):e236720.
47. Buchmeier MJ, Dalziel RG, Koolen MJ. Coronavirus-induced CNS disease: a model for virus-induced demyelination. J Neuroimmunol. 1988;20(2-3):111–6.
48. Cabirac GF, Soike KF, Zhang JY, Hoel K, Butunoi C, Cai GY, et al. Entry of coronavirus into primate CNS following peripheral infection. Microb Pathog. 1994;16(5):349–57.
49. Shabani Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol Belg. 2021;121(4):859–66.
50. Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404–18.
51. Perlman S, Jacobsen G, Afifi A. Spread of a neurotropic murine coronavirus into the CNS via the trigeminal and olfactory
nerves. Virology. 1989;170(2):556–60.
52. Baig AM, Sanders EC. Potential neuroinvasive pathways of SARS-CoV-2: Deciphering the spectrum of neurological
deficit seen in coronavirus disease-2019 (COVID-19). J Med Virol. 2020;92(10):1845–57.
53. Chaudhry F, Jageka C, Levy PD, Cerghet M, Lisak RP. Review of the COVID-19 risk in multiple sclerosis. J Cell Immunol. 2021;3(2):68–77.
54. Parrotta E, Kister I, Charvet L, Sammarco C, Saha V, Charlson RE, et al. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple
Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e835.
55. Etemadifar M, Sedaghat N, Aghababaee A, Kargaran PK, Maracy MR, Ganjalikhani-Hakemi M, et al. COVID-19 and the risk of relapse in multiple sclerosis patients: A fight with no bystander
effect? Mult Scler Relat Disord. 2021;51:102915.
56. Barzegar M, Vaheb S, Mirmosayyeb O, Afshari-Safavi A, Nehzat N, Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis?
A retrospective study. Mult Scler Relat Disord. 2021;52:102947.
57. Garjani A, Middleton RM, Hunter R, Tuite-Dalton KA, Coles A, Dobson R, et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented
by disease modifying therapies. Mult Scler Relat Disord. 2021;52:102939.
58. Dema M, Eixarch H, Villar LM, Montalban X, Espejo C. Immunosenescence in multiple sclerosis: the identification of new therapeutic targets. Autoimmun Rev. 2021;20(9):102893.
59. Fragoso YD, Pacheco FAS, Silveira GL, Oliveira RA, Carvalho VM, Martimbianco ALC. COVID-19 in a temporal relation to the onset of multiple sclerosis. Mult Scler Relat Disord. 2021;50:102863.
60. Palao M, Fernandez-Diaz E, Gracia-Gil J, Romero-Sanchez CM, Diaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45:102377.
61. Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021;22:100299.
62. Louapre C, Collongues N, Stankoff B, Giannesini C, Papeix C, Bensa C, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and
multiple sclerosis. JAMA Neurol. 2020;77(9):1079–88.
63. Montero-Escribano P, Matias-Guiu J, Gomez-Iglesias P, Porta-Etessam J, Pytel V, Matias-Guiu JA. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series
of 60 patients from Madrid, Spain. Mult Scler Relat Disord. 2020;42:102185.
64. Ghajarzadeh M, Mirmosayyeb O, Barzegar M, Nehzat N, Vaheb S, Shaygannejad V, et al. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated
on ocrelizumab during the pandemic. Mult Scler Relat Disord. 2020;43:102222.
65. Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in patients with multiple sclerosis: Associations with disease-modifying
therapies. CNS Drugs. 2021;35(3):317–30.
66. Foerch C, Friedauer L, Bauer B, Wolf T, Adam EH. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord. 2020;42:102180.
67. Barzegar M, Mirmosayyeb O, Gajarzadeh M, Afshari-Safavi A, Nehzat N, Vaheb S, et al. COVID-19 among patients with multiple sclerosis: A systematic review. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1001.
68. Amirian ES, Levy JK. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic
options for coronaviruses. One Health. 2020;9:100128.
69. National Multiple Sclerosis Society. COVID-19 vaccine guidance for people living with MS. Available from: https://www.nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance. Last updated: Jul 17, 2021; cited Jul 31, 2021
70. Centonze D, Rocca MA, Gasperini C, Kappos L, Hartung HP, Magyari M, et al. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert
consensus. J Neurol. 2021;268(11):3961–8.
71. Otero-Romero S, Ascherio A, Lebrun-Frenay C. Vaccinations in multiple sclerosis patients receiving disease-modifying drugs. Curr Opin Neurol. 2021;34(3):322–8.
72. Loebermann M, Winkelmann A, Hartung HP, Hengel H, Reisinger EC, Zettl UK. Vaccination against infection in patients with multiple sclerosis. Nat Rev Neurol. 2012;8(3):143–51.
73. Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J Neuroimmunol. 2021;356:577599.
74. Chilimuri S, Mantri N, Gongati S, Zahid M, Sun H. COVID-19 vaccine failure in a patient with multiple sclerosis on ocrelizumab. Vaccines (Basel). 2021;9(3):219.
75. Coyle PK, Gocke A, Vignos M, Newsome SD. Vaccine considerations for multiple sclerosis in the COVID-19 era. Adv Ther. 2021;38(7):3550–3588.
76. Etemadifar M, Sigari AA, Sedaghat N, Salari M, Nouri H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated
multiple sclerosis patient. Hum Vaccin Immunother. 2021;17(10):3481–3.
77. Buttari F, Bruno A, Dolcetti E, Azzolini F, Bellantonio P, Centonze D, et al. COVID-19 vaccines in multiple sclerosis treated with cladribine or ocrelizumab. Mult Scler Relat Disord. 2021;52:102983.
78. Achiron A, Dolev M, Menascu S, Zohar DN, Dreyer-Alster S, Miron S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February
2021. Mult Scler. 2021;27(6):864–70.
Matthew Topolski, 1 Second-year Medical Student. Lake Erie College of Osteopathic Medicine, Elmira, New
York 14901, United States.
Varun Soti, 2 Ph.D. Lake Erie College of Osteopathic Medicine, Elmira, New York 14901, United States.
About the Author: Matthew Topolski is a second-year medical student at Lake Erie College of Osteopathic
Medicine, Elmira, NY.
Correspondence: Varun Soti, Address: Lake Erie College of Osteopathic Medicine, Elmira, NY 14901,
USA. Email: vsoti@lecom.edu
Editor: Francisco J.Bonilla-Escobar
Student Editors: Eugenia M. Ramos-Dávila
Copyeditor: Ciara Egan
Proofreader: Nikoleta Tellios
Layout Editor: Lucianne A. Odiero
Process: Peer-reviewed
Cite as: Topolski M, Soti V. Effects of COVID-19 on Multiple Sclerosis Relapse: A Comprehensive
Review. Int J Med Stud. 2022 Apr-Jun;10(2):192-201.
Copyright © 2022 Matthew Topolski, Varun Soti
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 2, February 2022

