Values of Blood Glucose (A), Glycated Hemoglobin (Hb1Ac) (B) and Body Mass Index (BMI) (C) of Healthy Participants (CTL) versus Diabetic Participants (T2DM).
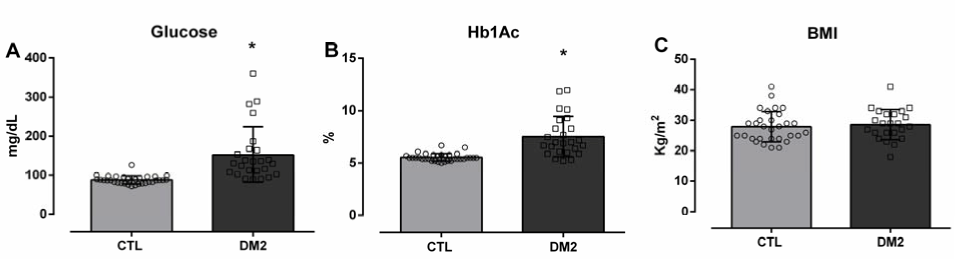
Legend: Values Expressed as mean±SD. *p<0.05 vs. CTL. Unpaired Student’s t-test.
Diogo P. Simões1, Matheus Moreira Perez2, Beatriz da C. Aguiar Alves3, Jéssica F. Araújo Encinas4, Joyce R. Santos Raimundo4, Catherine G. Costas Arcia5, Vanessa Lopes Mathia4, Maria I. Sacchi Mendonça4, Laura B. Mesiano Maifrino6, Neif Murad4, Fernando L. Affonso Fonseca7, Glaucia Luciano da Veiga3
doi: http://dx.doi.org/10.5195/ijms.2022.1306
Volume 10, Number 4: 387-394
Received 09 12 2021; Rev-request 07 07 2022; Rev-request 24 09 2022; Rev-recd 16 08 2022; Accepted 26 09 2022
ABSTRACT
Background:Diabetic nephropathy (DN) is a disorder affecting glomerular function that, histologically, is due to the presence of glomerulosclerosis accompanied with endothelial dysfunction of the afferent and efferent renal arterioles. Insulin resistance in diabetic patients is known to be one of the causes of endothelial dysfunction because it increases oxidative stress, and one of the main genes regulating the production pathways of reactive oxygen species is p66Shc. The aim of this study was to evaluate the p66Shc gene expression as a precocious biomarker of renal dysfunction in diabetic patients, using liquids samples of urine sediment and peripheral blood.
Methods:29 diabetic patients and 37 healthy donors were recruited from the Centro Universitário FMABC outpatient clinic. The RT-gPCR technique was applied to evaluate p66Shc gene expression in urine and peripheral blood samples from diabetic patients, which were compared with healthy donors.
Results:There was no significant expression of p66Shc gene in samples from diabetic patients compared with healthy donors. However, p66Shc expression in the blood samples of diabetics (0.02417±0.078652-ΔCT, n = 29) was 3.6 times higher than in healthy participants (0.00689±0.01758, n = 37) while in the urine samples, it was 1.48 times higher in diabetics group (0.02761±0.05412-ΔCT) than in CTL group (0.0186±0.02199).
Conclusion:There was no significant p66Shc gene expression in peripheral blood and urine samples of diabetic patients without kidney injury compared with healthy donors, although there is a tendency for this gene to participate in the oxidative imbalance present in diabetes.
Keywords: Diabetes Mellitus; p66Shc; Biomarker; Liquid biopsy (Source: MeSH-NLM).
Type II diabetes mellitus (T2DM) is a heterogeneous disorder defined by the presence of hyperglycemia due to the functional insufficiency of insulin’s action on its receptor.1,2 Currently, there has been an improved survival rate of diabetic patients and, in parallel, increased chances of developing chronic complications due to the long periods of exposure to hyperglycemia- among these complications is nephropathy, the main reason for the admission of patients to dialysis and transplantation programs.3,4
Diabetic nephropathy (DN) consists of a disorder that affects glomerular function, which, histologically, occurs due to the presence of glomerulosclerosis, a condition in which the basal membranes of the glomerular capillaries are thickened, and the mesangium, which surrounds the glomerular vessels, is increased due to the deposition of extra cellular matrix (ECM).5,6 This is an asymptomatic disease that is rarely identified in the early stages and is therefore considered potentially serious. It is detected between the moderate and late phases. This disease usually presents with three clinical phases that allow the classification of patients according to its progress.7,8
Endothelial nitric oxide synthase (eNOS) synthesis is impaired in patients with T2DM, due to factors such as hyperglycemia, hyperinsulinemia, and insulin resistance, which leads to one of the main factors involved in the pathophysiology of DN: a dysfunction of endothelial glomerular capillaries and afferent and efferent arterioles due to the increased production of reactive oxygen species (ROS) and reduced production of nitric oxide (NO). This condition results in vasoconstriction and endothelial oxidative stress that causes significant cell death and worsening of the condition of the patient with DN.9,10
The modulation of the oxidative stress process is performed by the p66Shc protein, which is an isoform of the SHC1 gene, located in the first chromosome. p66Shc acts on the endothelial cell by increasing the production of ROS through three different mechanisms, in the cell nucleus, in the cell membrane, and in the mitochondria, In the nucleus, p66Shc is mediated by Forkhead Box Sub-Group O (FOXO), resulting in decreased expression of the enzymes ROS-scavenging catalase (CAT) and manganese superoxide dismutase (MnSOD), both responsible for regulating ROS levels during cellular oxidative stress. In the mitochondria, p66Shc moves from the cytosol to the intermembrane space of the mitochondria, binding to cytochrome C, and becoming an oxidoreductase that catalyzes the production of hydrogen peroxide (H2O2).
The ROS generated by these mechanisms will activate mitochondrial permeability transition pores, culminating in organelle dysfunction, release of mitochondrial apoptotic factors (caspases), in cell apoptosis and finally in the generation of glomerular endothelial dysfunction and sclerosis.11,12 Considering the information mentioned above, this study evaluated the potential use of p66Shc gene expression in liquid biopsy using urine sediment and peripheral blood, before changes in classic biomarkers, such as creatinine or microalbuminuria. For this, a biomarker of oxidative stress pathway, which had already been studied by protein expression, was used.
The present study is a cross-sectional study. It was conducted in 2018 and early 2019, and the patients were treated at the medical specialties’ outpatient clinic of the Centro Universitário FMABC/FMABC. Patients who agreed to participate in the study were given a free and informed consent form (FICF). Blood samples were not stored and were discarded after the measurements were made. We conducted an interview to collect the volunteers’ personal data, as well as to measure height, body weight, and verify any and all medications used to treat diabetes and its comorbidities.
The studied groups were as follows: healthy individuals (CTL), healthy non-diabetic individuals without a family history of diabetes or kidney disease, and diabetic patients. The individuals who participated as healthy individuals or healthy non-diabetic individuals without a family history of diabetes or kidney disease were at least 21 years old, and non-smokers or users of illicit drugs. Diabetic patients (T2DM) group composed of patients diagnosed with type II diabetes mellitus (fasting glucose ≥140mg/dL and glycated hemoglobin greater than 7%) for at least five years, and preserved renal function (serum creatinine <1.3mg/dL and microalbuminuria <30mg/dL) with a minimum age of 21 years and undergoing treatment for T2DM. The inclusion criteria for T2DM group was expressed will to participate by the patient and diagnosed kidney disease (GFR <60mL/min/1.73m2 or GFR> 60mL/min/1.73m2) associated with at least one marker of parenchymal kidney damage (e.g. proteinuria> 15.0 mg/dL) present for at least three months. The exclusion criteria for the T2DM group was insulin dependent patient; hospitalization for any reason in the last 30 days; and patient with a history of chronic liver disease.
This study was approved by the Ethics Committee of the Centro Universitário FMABC (no. 2.302.284). The informed consent forms were given to the volunteers for completion prior to their participation. The present study was conducted in accordance with the relevant guidelines and regulations/ethical principles of the Declaration of Helsinki.
Determination of fasting plasma glucose was performed by assessing the concentration of glucose in the blood after a nocturnal fasting period. The automated enzymatic method was performed using fluoride serum. Evaluation of glycemic control was conducted with the values of fasting glucose, values above 140 mg/dL for glucose were considered altered.
Glycated hemoglobin (HbA1c) was determined using the low pressure liquid chromatography (LPLC) technique, using a DiaStat – Bio-Rad analyzer, which expressed the percentage of the total hemoglobin and evaluated the average blood glucose level, during a 3-month period. The collected material was 5 ml of whole blood with 1 ml of hemolyzed reagent. Values above 7% for HbA1c were considered altered.
Serum creatinine was measured by the ELISA method to assess the kidney function of patients. The standard methodology of the Clinical Analysis Laboratory of the Faculdade de Medicina do ABC was followed. The estimated GFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula.
Determination of microalbuminuria in isolated urine samples was performed by the Biosystems® immunoturbidimetry method (BioSystems S.A. Costa Brava, Barcelona - Spain). The reference value was up to 15 mg/L for normoalbuminuric and between 30mg and 300 mg/24h for microalbuminuria.
Determination of total plasma homocysteine was performed by the Abbott Diagnostics fluorescence polarization immunoassay. Plasma concentrations of total homocysteine were calculated by Abbott Axsym® and high values were considered to be those greater than 15 µmol/L, according to values proposed in a recent meta-analysis.
Quantification of cystatin C was performed using the Enzyme Linked Immunosorbent Assay (ELISA) method, Cystatin C Kit (Human), catalog ALX-850-292, and brand Enzo Life Sciences. This test was based on the identification of antigens by antibodies marked with an enzyme, which acted on its substrate and caused the color of the chromogen (colorless substance that when oxidized by the enzyme causes a change in its color) to change.
The total RNA in peripheral blood cells was extracted using the following method: total RNA was isolated from leukocytes contained in peripheral blood obtained through hemolysis by centrifugation at 2500 RPM for 15 minutes, using the TRIzol method (TRIzol LS Reagent, Thermo Fisher cat. no. 10296-010), and according to the manufacturer’s protocol. To extract total RNA from the urine sediment, samples (15 mL) were initially centrifuged at 2500 revolutions per minute (RPM) for 10 minutes at 4°C to obtain the urine sediment. The supernatant was then discarded and 1 ml of TRIzol was added to the cell pellet. The extraction process followed the standard protocol instructions for TRIzol. Total RNA concentration was estimated by spectrophotometric reading using a NanoDrop equipment (ThermoFisher Scientific - Waltham, Massachusetts, USA). Samples of total RNA (starting amount 1μg) obtained from peripheral blood and urine sediment were converted into cDNA using SSIII First Strand qPCR Supermix (Invitrogen, cat. no. 11752050), according to the manufacturer’s protocol. RT-qPCR, p66Shc gene expression was evaluated by real-time PCR (RT-qPCR). The specific primers for each selected gene were designed with the aid of the Primer3 Input 0.4.0 software program, available at http://frodo.wi.mit.edu/primer3/. The designed primer sequences were then checked for specificity by the Primer-BLAST program, available at http://www.ncbi.nlm.nih.gov/tools/primer-blast. To normalize the relative expression of the target genes, expression values of the reference gene RPL13A were used.
Sequence of specific primers and their amplicons: p66Shc, Forward: GCTGCATCCCAACGACAAAG, Reverse: GAGTCCGGGTGTTGAAGTCC, pb: 113
The results were expressed as mean ± standard deviation (SD). These were compared using unpaired student’s t-test and Mann-Whitney for non-parametric data. These analyses were performed with the aid of the computer program GraphPad Prism (GraphPad, version 7.0, USA). The significance level was set at 5% (descriptive p <0.05). The sample size was determined by calculations performed in the computer program GPower 3.1.
A total of 66 volunteers were evaluated, of which 37 healthy participants - CTL and 29 patients with T2DM. Within the CTL group, there were 55% female participants and 45% male participants. The mean age was 45±14 years with a predominance of Caucasian ethnicity (89%), and 5% of the total were hypertensive (Table 1).
Table 1.Anthropometric Data of Participants in the Healthy Group (CTL)
| Parameters | |
|---|---|
| Gender (%) | |
| Female | 55 |
| Male | 45 |
| Age (mean±SD) years | 45±14 |
| Ethnicity (%) | |
| Black/Brown | 11 |
| Caucasian | 89 |
| Arterial Hypertension (%) | |
| Yes | 5 |
| No | 95 |
| Don&t know | 0 |
In the T2DM group, 60% of the participants were females and 40% were males. The mean age was 63±8 years and the predominant ethnicity was Caucasian (75%). The majority of the participants in this group reported having arterial hypertension (60%), 20% were not hypertensive and another 20% reported not knowing this information. When the time since T2DM diagnosis was evaluated, was found that 80% of the participants had been diabetic for at least 5 years, 5% for 5 to 10 years and 15% for more than 10 years (Table 2).
Table 2.Anthropometric Data of Participants in the Diabetic Group (T2DM)
| Parameters | |
|---|---|
| Gender (%) | |
| Female | 60 |
| Male | 40 |
| Age (mean±SD) years | 63±8 |
| Ethnicity (%) | |
| Black/Brown | 25 |
| Caucasian | 75 |
| Arterial Hypertension (%) | |
| Yes | 60 |
| No | 20 |
| Don't know | 20 |
| Time of disease (%) | |
| 0-5 years | 80 |
| 5-10 years | 5 |
| >10 years | 15 |
To characterize the studied sample, blood glucose measurements of the CTL and T2DM groups (87±11 vs. 152±71 mg/dL, *p<0.05) as well as Hb1Ac (5.5±0.4 vs. 7.5±1.9%, *p<0.05) were performed. A statistical difference between the groups, due to high values of these biochemical markers, was expected and observed. BMI values were compared between groups (CTL: 27±5 vs. T2DM: 28±5 Kg/m2) (Figure 1).
Figure 1.Values of Blood Glucose (A), Glycated Hemoglobin (Hb1Ac) (B) and Body Mass Index (BMI) (C) of Healthy Participants (CTL) versus Diabetic Participants (T2DM).

Figure 2 shows the evaluations of classic biochemical markers of renal function. Alterations in the values of plasma creatinine (A) (CTL 0.80±0.20 vs. T2DM 0.87±0.29 mg/dL), urinary creatinine (B) (CTL 134±85 vs. 130±69 mg/dL), urea (C) (CTL 32±12 vs. T2DM 53±83 mg/dL), proteinuria (D) (CTL 12.2±10.2 vs. 23.1±48.1 mg/dL) and GFR (F) data were not observed. We only verified alterations in the values of microalbuminuria (D) (CTL 20.3±37.0 vs. T2DM 23.4±24.7 mg/L, *p<0.05).
Figure 2.Values for Plasma Creatinine (A), Urinary Creatinine (B), Urea (C), Microalbuminuria (D), Proteinuria (E) and Glomerular Filtration Rate (GFR) (F) of Healthy Participants (CTL) versus Diabetic Participants (T2DM).
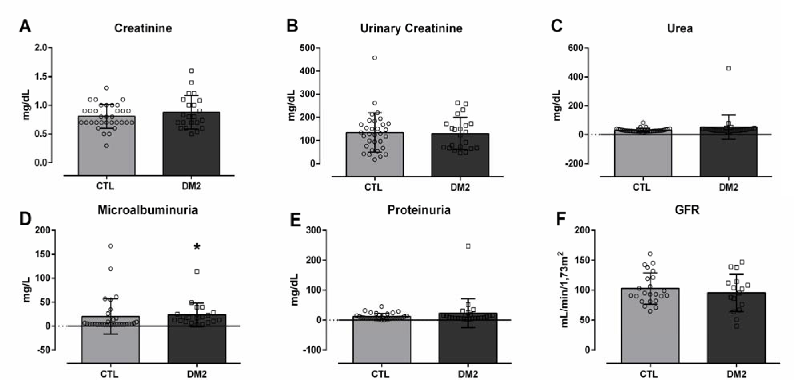
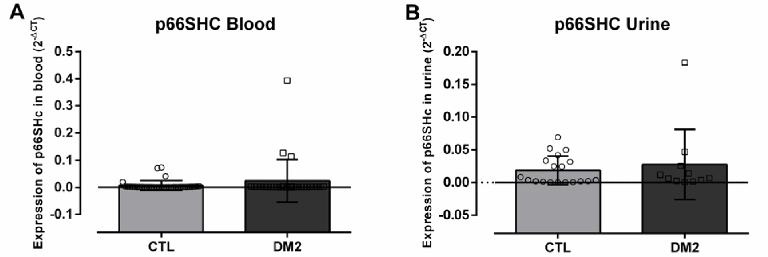
Figure 3 illustrates p66Shc gene expression in blood (A) and urine (B) samples. It was observed that there was no statistical difference in the expression of this gene between the CTL group and T2DM group. However, p66Shc expression in blood was 3.6 times higher in diabetics (T2DM 0.024±0.079) than in healthy participants (CTL 0.0069±0.0176 2-ΔCT). In urine, p66Shc expression was 1.48 times higher in diabetics (0.0276±0.0541) than in CTL (0.0186±0.0219 2-ΔCT).
Figure 3.p66Shc Gene Expression Values (2-ΔCT) in Healthy Participants (CTL) Compared to Diabetic Participants (T2DM) in Blood (A) and Urine (B) Samples.
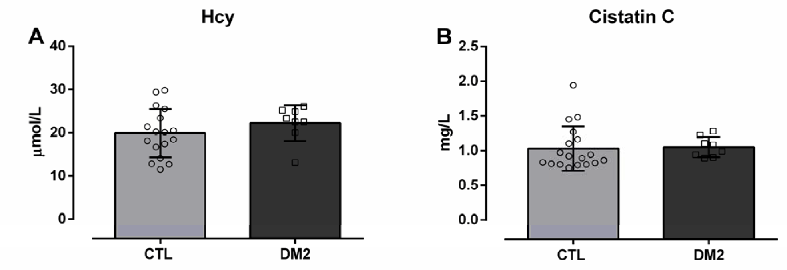
When measuring Hcy and cystatin C concentrations, no significant alterations between groups were identified: Hcy T2DM (22.20±4.15 µmol/L) and CTL (19.90±5.61 µmol/L), cystatin C (T2DM 1.05±0.15 vs. CTL 1.02±0.14 mg/dL) (Figure 4).
Figure 4.Representative Graphs Referring to the Measurement of Homocysteine (A) and Cystatin C (B) of Healthy Participants (CTL) versus Diabetic Participants (T2DM).
The concept of liquid biopsy is based on the use of liquid/fluid samples (especially from peripheral blood or urine) to detect early changes in the expression of a gene of choice, through the evaluation of cell-free nucleic acids. This is a new approach that has already been studied in cancer and is able to indirectly reflect the future expression of proteins involved in the formation of tumors or changes in tissue function. Furthermore, the fundamental idea of this method is to be less invasive than traditional disease probing methods. This method still needs to be standardized for the consolidation of its diagnostic and/or prognostic use, and therefore, the efforts for this elucidation are valid. This study evaluated a biomarker that actively participates in the oxidative stress pathway, to propose an early marker of renal changes in diabetics patients.13–15
Oxidative stress in diabetic patients is higher when compared to healthy patients, since the formation of intracellular ROS - considered a common route in renal injury induced by hyperglycemia - is greater in these patients and increases proportionally with the development of the disease.16 As previously described, p66Shc is a protein responsible for modulating the production of mitochondrial ROS, causing the stressed cell to produce more ROS, providing positive feedback that results in cell apoptosis. Despite the studies identifying this activation of p66Shc associated with the pathophysiology of DN in endothelial cells, this study evaluated whether this alteration also occurred in peripheral blood leukocytes and urinary sediment cells, in view of the ease and importance of using liquid biopsy for monitoring of patients. Liquid biopsy, originally studied in oncology, consists of isolating circulating cells as a source of genomic and proteomic information.17
The increase in p66Shc gene expression in this study was not significant. However, further analysis of the research showed that the p66Shc gene expression in the blood was 3.6 times higher in diabetics compared to healthy individuals, and in urine, this difference was 1.48 times greater under the same comparison. This suggests that in as number of patients increases, a significant increase in this gene expression would be seen. The study of p66Shc gene expression in diabetic patients without diagnosed kidney injury had not been explored, at least to our knowledge.
The data of this study showed that all diabetic patients had high glucose and glycated hemoglobin levels, adequately supporting the purpose of this research. At the same time, classic markers of the onset of kidney disease, creatinine, proteinuria, and microalbuminuria were evaluated, and GFR was calculated. In all these measurements, it was observed that the patients did not have kidney disease prior to the evaluations.
As known, cystatin C is a current and accurate marker of the assessment of initial renal function loss, as it is freely filtered by the glomerulus and subsequently reabsorbed in the proximal tubule. So, the serum cystatin C levels reflect glomerular filtration- its increase in serum means a reduction in GFR.18 The patients in this study did not show any increase in the measurement of this marker, confirming that the diabetic group did not have nephropathy.
The participants with T2DM did not show an increase in Hcy measurements, which indicated the absence of endothelial injury. In this study, there was a subtle alteration in p66Shc gene expression, without significant changes in Hcy levels. The relationship between p66Shc overexpression and increased Hcy levels has been previously described in patients with confirmed endothelial dysfunction. The main cause of this relationship is closely linked to DNA methylation, promoted by p66Shc.19,20 Our data suggest that the increase in p66Shc expression is the mechanism responsible for the initiation of the deregulation of Hcy synthesis. We believe that the consolidation of the over-expression of this gene increases DNA methylation and causes, in later stages of T2DM, irreversible endothelial changes, followed by kidney damage. On the other hand, when there are high levels of the Hcy precursor, S-Adenosilhomocysteine (SAH), there is an increase in the production of ROS and in the expression of p66Shc in endothelial cells, that is, there is self-regulation between the expulsion of this gene and the increase in Hcy.21
The relationship between p66Shc expression and diabetic nephropathy is evident, as shown in studies in mice. These studies have shown that the deletion of this gene prevents endothelial dysfunction induced by hyperglycemia, in addition to reducing the oxidative stress of cells, which prevented the alteration of renal structure and function in these animals.22–24 The endothelial and myoblastic cells of p66Shc knockout mice demonstrated a lower rate of apoptosis in ischemic conditions, thus proving the role of p66Shc in cell survival in response to hypoxia.25 Another protein involved in renal damage to DN is the hypoxia-inducible factor (HIF-1α). The relationship between p66Shc and HIF-1α has been described by proposing a pathway in which HIF-1α, stimulated by T-cell hypoxia, activates p66Shc, which contributes to the release of extracellular vascular endothelial growth factor (VEGF) and, is one of the responses to low oxygenation mediated by HIF-1α. In addition, p66Shc itself is stimulated by oxidative stress induced by hypoxia, and triggers cell apoptosis.26 Our study identified the subtle elevation of p66Shc gene expression in diabetic patients who, as previously described, are in oxidative imbalance due to hyperglycemia, hyperinsulinemia, and insulin resistance. Therefore, it can be suggested that the pathway explained above is activated.9
Expression of the p66Shc protein in peripheral blood and in renal tissue of diabetic patients had already been studied, under conditions of already established kidney injury. The authors found an increase in the expression of the p66Shc protein and suggested that the evaluation of its expression in peripheral blood could be used as a potential biomarker of the progression of kidney injury mediated by increased oxidative stress.27
Considering that the oxidative stress pathway is activated by hyperglycemia, we suggest that in our patients there is a synergism between the hyperexpression of markers against decapentaplegic homolog 1 (SMAD1), which are intracellular proteins capable of regulating transcription factors and expression of target genes, associated with that of p66Shc, with the first promoting mesangial expansion and the second mediating oxidative imbalance. Positive feedback between these two pathways may be responsible for the gradual and silent loss of kidney function. Therefore, we suggest that minor changes in p66Shc gene expression may signal the dysregulation of the oxidative system and, thus, lead to late kidney damage.21,23,28,29
This study showed that there was no significant p66Shc gene expression in peripheral blood and urine samples of diabetic patients without kidney injury in comparison with healthy donors. However, in our experimental conditions p66Shc gene expression is slightly elevated in T2DM group. There was no association between increased gene expression and the other laboratory variables that were studied. We believe that increasing the number of patients may elucidate the viability of the data.
The limitation of this study was the small sample size and the difficulty in assessing gene expression in urine samples. We are certain that the use of specific extraction kits for samples of low cellularity, such as urine samples, will facilitate this type of study in this biological matrix.
Título: Estudo transversal da expressão do gene p66Shc em biópsia líquida de pacientes diabéticos. É possível prever o início da doença renal? O objetivo deste estudo foi avaliar a expressão do gene p66Shc como um possível biomarcador precoce de disfunção renal em pacientes com diabetes, utilizando amostras líquidas de sedimento urinário e sangue periférico. Foram avaliados 29 pacientes diabéticos e 37 doadores saudáveis, estes foram recrutados no ambulatório do Centro Universitário FMABC. Foi avaliada a expressão do gene p66Shc pela técnica de RT-qPCR em amostras de urina e sangue periférico de pacientes diabéticos e foram comparadas com doadores saudáveis. Não foi observado alteração da expressão do gene p66Shc em amostras de pacientes diabéticos em comparação com doadores saudáveis. No entanto, a expressão de p66Shc no sangue de diabéticos foi 3,6 vezes maior em diabéticos (0,02417±0,078652-ΔCT, n=29) do que em participantes saudáveis (0,00689±0,01758, n=37) e na urina foi 1,48 vezes maior no grupo de diabéticos (0,02761±0,05412-ΔCT) do que no grupo CTL (0,0186±0,02199). Portanto, não houve expressão significativa do gene p66Shc em amostras de sangue periférico e urina de pacientes diabéticos sem lesão renal em comparação com doadores saudáveis, embora haja uma tendência desse gene participar do desequilíbrio oxidativo presente no diabetes.
We thank the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), for financial support in this study
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: DPS, FLAF, GLV. Data Curation: MMP, BCAA, FLAF, GLV. Formal Analysis: GLV. Funding Acquisition: DPS, FLAF, GLV. Investigation: DPS, MMP, BCAA, JFAE, JRSR, CGCA, VLM, MISM, LAMM, NM. Methodology: MMP, BCAA, JFAE, JRSR, CGCA, VLM. Project Administration: DPS, FLAF, GLV. Resources: MMP, FLAF, GLV. Software: BCAA, GLV. Supervision: FLAF, GLV. Validation: MMP, BCAA, FLAF, GLV. Visualization: DPS, MMP, BCAA, JFAE, JRSR, CGCA, VLM, MISM, LAMM, NM, FLAF, GLV. Writing – Original Draft Preparation: DPS, FLAF, GLV. Writing – Review & Editing: DPS, MMP, FLAF, GLV.
1. de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2014;37(10):2843–63.
2. Maraschin JeF. Classification of diabetes. Adv Exp Med Biol. 2012;771:12–9.
3. Li YR, Tsai SS, Lin YS, Chung CM, Chen ST, Sun JH, et al. Moderate- to high-intensity statins for secondary prevention in patients with type 2 diabetes mellitus on dialysis after acute myocardial infarction. Diabetol Metab Syndr. 2017;9:71.
4. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44 Suppl 2: S14–21.
5. Selby JV, FitzSimmons SC, Newman JM, Katz PP, Sepe S, Showstack J. The natural history and epidemiology of diabetic nephropathy. Implications for prevention and control. JAMA. 1990;263(14):1954–60.
6. Ma J, Wu H, Zhao CY, Panchapakesan U, Pollock C, Chadban SJ. Requirement for TLR2 in the development of albuminuria, inflammation and fibrosis in experimental diabetic nephropathy. Int J Clin Exp Pathol. 2014;7(2):481–95.
7. Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care. 2010;33(11):2484–5.
8. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28.
9. Nacci C, Tarquinio M, Montagnani M. Molecular and clinical aspects of endothelial dysfunction in diabetes. Intern Emerg Med. 2009;4(2):107–16.
10. Murkamilov IT, Sabirov IS, Fomin VV, Yusupov FA. [Endothelial dysfunction and arterial wall stiffness: New targets in diabetic nephropathy]. Ter Arkh. 2017;89(10):87–94.
11. Fadini GP, Albiero M, Menegazzo L, Boscaro E, Pagnin E, Iori E, et al. The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes. 2010;59(9):2306–14.
12. Magenta A, Greco S, Capogrossi MC, Gaetano C, Martelli F. Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction. Biomed Res Int. 2014;2014:193095.
13. Poulet G, Massias J, Taly V. Liquid Biopsy: General Concepts. Acta Cytol. 2019;63(6):449–55.
14. Alix-Panabières C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016;6(5):479–91.
15. Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16(7):409–24.
16. Pan HZ, Zhang L, Guo MY, Sui H, Li H, Wu WH, et al. The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol. 2010;47 Suppl 1: 71–6.
17. Palmirotta R, Lovero D, Cafforio P, Felici C, Mannavola F, Pellè E, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol. 2018;10:1758835918794630.
18. Kim SS, Song SH, Kim IJ, Jeon YK, Kim BH, Kwak IS, et al. Urinary cystatin C and tubular proteinuria predict progression of diabetic nephropathy. Diabetes Care. 2013;36(3):656–61.
19. Grimaldi V, Vietri MT, Schiano C, Picascia A, De Pascale MR, Fiorito C, et al. Epigenetic reprogramming in atherosclerosis. Curr Atheroscler Rep. 2015;17(2):476.
20. Kim CS, Kim YR, Naqvi A, Kumar S, Hoffman TA, Jung SB, et al. Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovasc Res. 2011;92(3):466–75.
21. Xiao Y, Xia J, Cheng J, Huang H, Zhou Y, Yang X, et al. Inhibition of S-Adenosylhomocysteine Hydrolase Induces Endothelial Dysfunction via Epigenetic Regulation of p66shc-Mediated Oxidative Stress Pathway. Circulation. 2019;139(19):2260–77.
22. Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, et al. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104(12):5217–22.
23. Menini S, Iacobini C, Ricci C, Oddi G, Pesce C, Pugliese F, et al. Ablation of the gene encoding p66Shc protects mice against AGE-induced glomerulopathy by preventing oxidant-dependent tissue injury and further AGE accumulation. Diabetologia. 2007;50(9):1997–2007.
24. Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, et al. Erratum. Deletion of p66. Diabetes. 2018;67(1):165.
25. Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109(23):2917–23.
26. Naldini A, Morena E, Pucci A, Pellegrini M, Baldari CT, Pelicci PG, et al. The adaptor protein p66Shc is a positive regulator in the angiogenic response induced by hypoxic T cells. J Leukoc Biol. 2010;87(3):365–9.
27. Xu X, Zhu X, Ma M, Han Y, Hu C, Yuan S, et al. p66Shc: A novel biomarker of tubular oxidative injury in patients with diabetic nephropathy. Sci Rep. 2016;6:29302.
28. Veiga G, Alves B, Perez M, et al. NGAL and SMAD1 gene expression in the early detection of diabetic nephropathy by liquid biopsy. J Clin Pathol. 2020;73(11):713–721.
29. Matsubara T, Araki M, Abe H, Ueda O, Jishage K, Mima A, et al. Bone Morphogenetic Protein 4 and Smad1 Mediate Extracellular Matrix Production in the Development of Diabetic Nephropathy. Diabetes. 2015;64(8):2978–90.
Diogo P. Simões, 1 Sixth-year Medical Student. Universidade Municipal de São Caetano do Sul/USCS - São Caetano do Sul, Brazil. Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Matheus Moreira Perez, 2 MsD. Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Beatriz da C. Aguiar Alves, 3 PhD. Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Jéssica F. Araújo Encinas, 4 Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Joyce R. Santos Raimundo, 4 Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Catherine G. Costas Arcia, 5 Centro Universitário São Camilo - São Paulo, SP, Brazil. Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Vanessa Lopes Mathia, 4 Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Maria I. Sacchi Mendonça, 4 Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Laura B. Mesiano Maifrino, 6 Universidade São Judas Tadeu, Programa de Pós-Graduação, São Paulo, SP, Brazil.
Neif Murad, 4 Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
Fernando L. Affonso Fonseca, 7 PhD. Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil. Departamento de Ciências Farmacêuticas, Universidade Federal de São Paulo, Campus Diadema, Diadema, Brazil.
Glaucia Luciano da Veiga, 3 PhD. Laboratório de Análises Clínicas - Centro Universitário Saúde ABC/FMABC – Santo André, Brazil.
About the Author: Diogo P. Simões is currently a sixth-year medical student of Universidade Municipal de São Caetano do Sul/USCS - São Caetano do Sul, Brazil. He received training in laboratorial analysis in the Molecular Biology and helped in several other studies.
Correspondence: Diogo P. Simões. Address: Santo Antonio, 50 - Centro, São Caetano do Sul - SP, 09521-160, Brazil. Email: grlveiga@gmail.com
Editor: Mohammad Amin Khazeei Tabari; Student Editors: Rahul Abraham & Hooman Khoshhal; Copyeditor: Sebastian Diebel; Proofreader: Laeeqa Manji; Layout Editor: Ana Maria Morales; Process: Peer-reviewed
Cite as Simões DP, Perez MM, Alves BCA, Encinas JFA, Raimundo JRS, Arcia CGC, et al. A Cross-Sectional Study of p66Shc Gene Expression in Liquid Biopsy of Diabetic Patients. Is It Possible to Predict the Onset of Renal Disease?. Int J Med Stud. 2022 Oct-Dec;10(4):387-94.
Copyright © 2022 Diogo P. Simões, Matheus Moreira Perez, Beatriz da C. Aguiar Alves, Jéssica F. Araújo Encinas, Joyce R. Santos Raimundo, Catherine G. Costas Arcia, Vanessa Lopes Mathia, Maria I. Sacchi Mendonça, Laura B. Mesiano Maifrino, Neif Murad, Fernando L. Affonso Fonseca, Glaucia Luciano da Veiga
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 4, December 2022