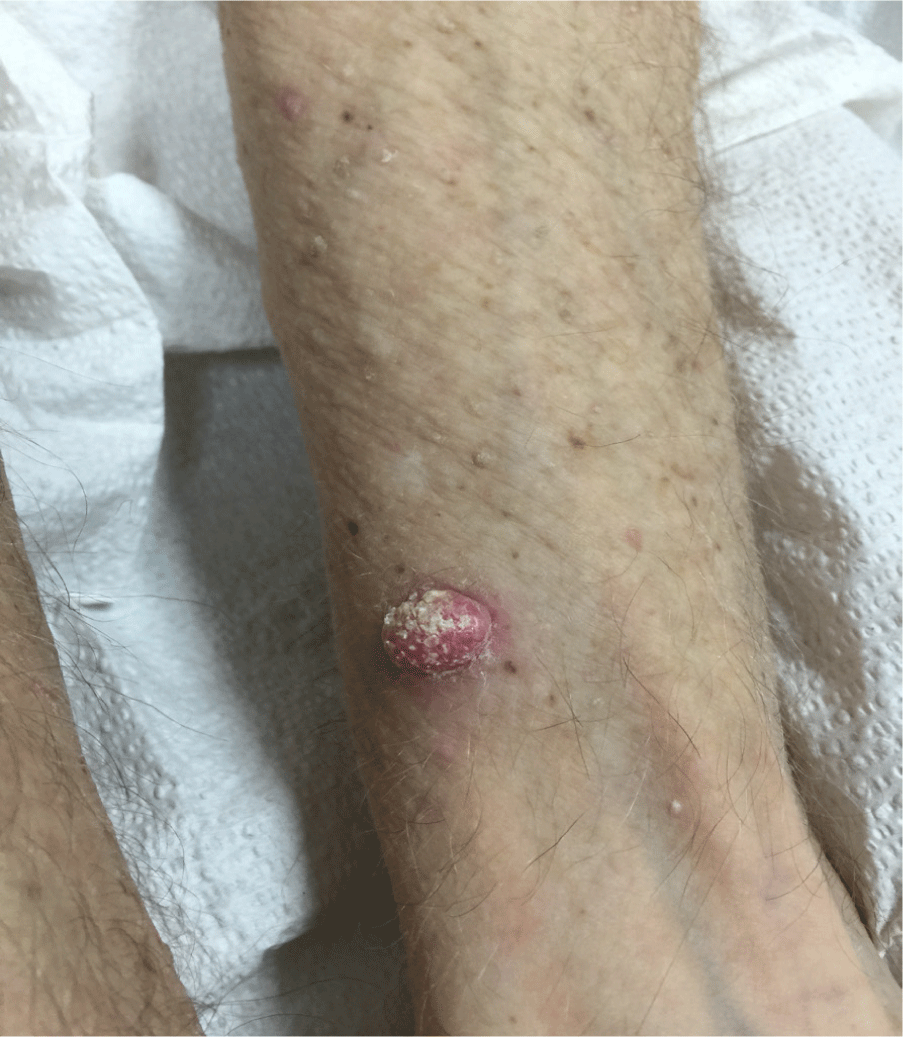
Squamous Cell Carcinoma, Keratoacanthoma Type on the patient's left volar forearm.
Siddhant Thukral1, Lloyd E. King Jr2, David H. Horowitz2
doi: http://dx.doi.org/10.5195/ijms.2015.137
Volume 3, Number 3: 155-158
Received 19 08 2015: Accepted 11 11 2015
ABSTRACT
Background:Treatment for adult Philadelphia chromosome+ acute lymphoblastic leukemia includes using dasatinib, a tyrosine kinase inhibitor. Cutaneous squamous cell carcinomas and keratoacanthomas are common findings in patients treated with BRAF inhibitors of tyrosine kinases. No documentation of dasatinib inducing multiple keratoacanthomas, squamous cell carcinomas type during treatment of Philadelphia chromosome+ acute lymphoblastic leukemia is currently available.
Case:A 77-year-old Caucasian male presented to the dermatology clinic two months after starting treatment with dasatinib for Philadelphia chromosome positive+ acute lymphoblastic leukemia. Biopsies confirmed the lesions on the arms, chest, legs and back as keratoacanthoma (KA) type of squamous cell carcinomas (SCCs). The cutaneous lesions were surgically removed and no new or recurrent lesions were detected since their initial rapid onset despite continued dasatinib therapy.
Conclusion:This report of the rapid onset of keratoacanthoma type squamous cell carcinomas in a patient with Philadelphia chromosome+ acute lymphoblastic leukemia treated with dasatinib is presumed to be the first due to the rarity of adult Philadelphia chromosome+ acute lymphoblastic leukemia. This report documents another tyrosine kinase inhibitor that is associated with the eruption of keratoacanthomas, and adds to the literature regarding the regularity of this relatively common side effect, which may have treatment other than surgery if only a few lesions are present.
Keywords: Dasatinib; Keratoacanthoma; Carcinoma, Squamous Cell; Lymphoblastic Leukemia, Acute; Bcr-Abl Tyrosine Kinase.
Acute lymphoblastic leukemia (ALL) is a malignant disorder of lymphoid progenitor cells with unknown pathogenicity diagnosed in between 3,000 to 4,000 people in the U.S each year, two-thirds of them are children.1–2 In adult ALL, the most frequent chromosomal translocation is t(9;22), known as the Philadelphia chromosome, which binds breakpoint cluster (BCR) signalling proteins to ABL tyrosine kinases (TKs) resulting in uncontrollable ABL tyrosine kinase (TK) activity.1–3
In the past two decades, tyrosine kinase inhibitors (TKIs) that specifically inhibit certain TKs, such as those involved in ALL's pathogenesis, have successfully treated ALL.4–5 Dasatinib, an orally available second-generation ABL kinase inhibitor, is approved to treat adults with Philadelphia chromosome positive (Ph+) ALL who developed resistance to or intolerance of other ABL TKIs such as imatinib (Gleevec®).3–6
Multiple factors such as exposure to ionizing radiation, chemical agents, UV radiation, human papilloma virus, and immunosuppression can predispose patients to develop different types of cutaneous squamous cell carcinomas (SCCs).5–7 Keratoacanthomas (KAs) are currently classified as one type of cutaneous squamous cell carcinoma (SCC), but this concept is controversial as another opinion is that it is a distinct self-resolving squamoproliferative lesion that may regress due to upregulation of the cell death/apoptosis pathway.8 Clinically, KAs may be a single or multiple rapidly developing crateriform lesions in a few weeks or months and may spontaneously resolve in 6 months without any treatment (Figure 1). Biopsies of KAs may or may not have different diagnostic features from other types of cutaneous SCCs such as those arising from the much more common actinic keratosis and in situ squamous cell carcinomas. A clinical history of rapid onset of such lesions within a few months is a valuable clue to their diagnosis and establishing their prognosis. Herein we report a case of a patient who developed multiple KAs within two months after starting treatment with dasatinib for Ph+ ALL. Sometimes KAs arise due to hyperactive epidermal growth factor receptor tyrosine kinases that subsequently activate the RAS and MAPK signalling pathways.5–9 However, the incidence and treatment of KA type SCCs induced by TKIs are not well defined, but investigations to clarify if KAs are SCCs and distinct from a molecular perspective are on-going.6–8 A signed copy of consent to publish this manuscript was obtained from the patient.
Figure 1.Squamous Cell Carcinoma, Keratoacanthoma Type on the patient's left volar forearm.

A 77-year-old Caucasian male presented to the dermatology clinic for sudden onset of nodular skin lesions found on the arms, legs, back, and chest. The patient showed no significant evidence of severe sun exposure or any ionizing radiation that would predispose him to develop these lesions. He had a medical history of a malignant melanoma on the left side of his upper back treated 9 years prior to his dermatology clinic visit. He had no family history of ALL, but his mother had treatment for a basal cell carcinoma. The diagnosis of Ph+ ALL was confirmed two months prior to his initial dermatology clinic visit and his skin lesions were first noticed weeks after starting treatment for Ph+ ALL. His oncologist started treatment with oral 400 mg tablet of antiviral drug Acyclovir taken twice daily, oral multi BCR/ABL TKI dasatinib (Sprycel®) 140 mg tablet taken once daily, and oral ondansetron (Zofran®) 8 mg tablet to be taken as needed. Within two months after starting this treatment, the patient achieved molecular remission of Ph+ ALL with undetectable BCR-ABL. The patient maintained the treatment regimen throughout his oncology clinic visits. While on this treatment regimen that included dasatinib, the patient developed mild anemia (RBC 3.50 M/uL). However, the patient did not have any fevers, chills, night sweats, nausea, vomiting, abdominal pain, chest pain, shortness of breath, or skin rash. The patient's oncologist confirmed his adherence to medication with regularly scheduled visits. The patient's wife also confirmed compliance with treatment regimen, and the patient was overall happy with the treatment results.
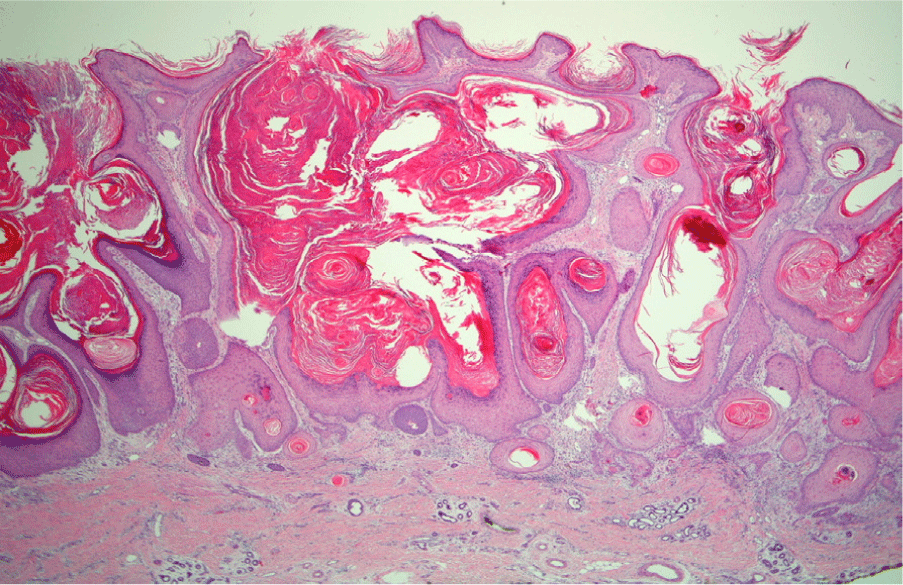
The patient's skin lesions were nodular, erythematous, hyper-keratotic, and first observed two weeks after starting treatment with dasatinib (Figure 1). The initial shave biopsy in the dermatology clinic was a 13 × 13 × 6mm tan nodule on the left volar forearm (Figure 1) to identify if it was a KA type of SCC, dermatitis medicamentosa, leukemia cutis, basal cell carcinoma or, less likely, a recurrent malignant melanoma. The dermatopathologist confirmed this nodule was a KA variant of SCC with clear margins (Figure 2). On the patient's next dermatology clinic visit one week following the initial skin biopsy, a repeat skin biopsy on the left superior forearm confirmed the second lesion was also a KA variant of SCC. These two KAs with typical biopsy features and clinical multiple other KAs (23 in total), their rapid clinical onset, and previous reports of KAs in patients treated with other TKIs (sorafenib, nilotinib) are consistent with the patient developing KAs induced by dasatinib used to treat Ph+ ALL.
Figure 2.Biopsy specimen confirmation of Squamous Cell Carcinoma, Keratoacanthoma type from Patient's Left Volar Forearm.
On follow-up visit three weeks after the preceding biopsy, no new lesions were detected and existing lesions showed hyper-keratotic regression typical of KAs. Due to a risk of slow healing and excessive bleeding, a referral to a Mohs surgeon was made to excise the 20 remaining KAs below the patient's waist. The three remaining KAs above the waist were excised in the dermatology clinic with sufficient time between visits to prevent slow healing and/or bleeding complications. The oncologist recommended that the patient continue 140 mg dasatinib and stop taking Acyclovir. An initial management challenge was to determine which physician should be managing his skin lesions – his oncologist, the dermatologist, or the Mohs surgeon? After initially cancelling his dermatology clinic appointment, convenient appointment times were made for the patient to see the oncologist and the dermatologist. Both clinicians and the patient are satisfied with the outcome of treatment and he continues close monitoring to detect new lesions or other adverse events. The patient was eager to achieve resolution of his skin lesions so he was compliant with all treatments, diagnostic tests, and has continued dasatinib for treatment of his Ph+ ALL.
Keratoacanthomas (KAs) are commonly thought to be a low-grade skin cancer or a distinct self-resolving squamoproliferative lesion that is very similar phenotypically to squamous cell carcinoma (SCC) and originates in the skin's pilosebaceous glands or hair follicles.10 KAs typically and rapidly grow a few weeks to a few months, as a crateriform lesion with hyperkeratotic regression.10 The patient's rapidly growing lesions were scattered throughout his chest, back, and extremities with no correlation between their location and intense sun exposure sites starting two months after treatment for Ph+ acute lymphoblastic leukemia (ALL) by his oncologist who referred the patient to the dermatology clinic. A shave biopsy was determined to be the ideal diagnostic approach. While multiple shave biopsies are diagnostically useful, only one or two biopsies were performed per visit to insure that the patient would not bleed excessively. The Mohs technique was determined to be the most appropriate for lesions below the patient's waist due to its accuracy and minimally invasive approach.
To define the etiology of the sudden and aggressive appearing KAs, the focus changed to a family history of skin cancers, a comorbid neoplasm related to Ph+ ALL, or possible adverse reaction to one of his medications including the tyrosine kinase inhibitor, dasatinib, which has an extensive list of cutaneous side effects including rashes, redness, peeling, but currently there is no reference of cutaneous SCC, KA type (Sprycel. Available from: http://www.sprycel.com/side-effects, updated 2015 August 15; cited 2015 August 16).
The current TKI literature implies a relationship between eruptive epitheliomas such as KAs and the TKIs sorafenib and nilotinib.5,10–11 There are multiple mutations associated with Ph+ ALL and interest is focused on the signaling pathways are targeted in the pathogenesis of KA type of SCC by dasatinib and other TKIs.12 One study documented a patient developing multiple cutaneous SCCs (KAs) shortly after starting treatment with nilotinib for chronic myeloid leukemia (CML).5 Multiple patients treated for solid tumors with sorafenib also developed multiple KA type lesions within few weeks to months after starting treatment.10–11 Our in-depth literature review did not find any documented cases of a Ph+ ALL with a patient with KA type eruptive epitheliomas or SCCs after starting therapies using dasatinib. Treatment options for patients treated with TKIs included a CML patient who developed KA type SCC lesions after starting treatment with nilotinib and the number of new lesions decreased after retinoid treatment and all existing SCCs were surgically excised.5 The induction of KAs may be TKI specific as patients with KAs treated with sorafenib for solid tumors had regression of existing KA, SCC type after switching to a different TKI (sunitinib) or discontinuing sorafenib.10–11
The mechanisms involved in causing the SCC, KA type lesions in our patient are not yet documented. Since no prior report was found to document eruptive epitheliomas, KAs, or SCC types were induced by dasatinib in the treatment of Ph+ ALL, we presume that this case is the first of its kind.
The treatment of Ph+ ALL with dasatinib has been associated with numerous different systemic and skin adverse events; however, its association with the rapid onset of keratoacanthoma type, squamous cell carcinomas in this rare form of ALL is not previously documented. With increased usage of dasatinib to treat adult Ph+ ALL, it is important to recognize its association with the possible rapid development of eruptive epitheliomas, keratoacanthoma type.
Key Points:We would like to thank Jacob P. Van Houten, MD/PhD candidate at Vanderbilt Department of Biomedical Informatics, for his constructive feedback on this case report and Jeffrey Zwerner MD/PhD at Vanderbilt Division of Dermatology for his photo microscopy of the biopsied SCC, KA type lesion. We would also like to thank our patient for his support throughout this process and his willingness to share his story and pictures of lesions for the advancement of medical knowledge.
The author has no funding, financial relationships or conflicts of interest to disclose.
Conception and design the work/idea: LEK, Collect data/obtaining results, Analysis and interpretation of data, Approval of the final version: ST, LEK, DHH. Write the manuscript: ST. Critical revision of the manuscript, Administrative or technical advice: LEK, DHH. Contribution of patients or study material: LEK.
1. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008 Mar 22;371 (9617):1030–43.
2. Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998 Aug 27;339 (9):605–15.
3. Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette Ret al . N Engl J Med. 2006 Jun 15;354 (24):2531–41.
4. Hoelzer D, Gökbuget N, Ottmann O, Pui CH, Relling MV, Appelbaum FRet al . Acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2002:162–92.
5. Peters P, Rabbolini D, Sinnya S, Khosrotehrani K, Wagner G. Multiple squamous cell carcinomas following introduction of nilotinib. Clin Exp Dermatol. 2014 Oct;39 (7):791–4.
6. Fielding AK. Current treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2010 Jan;95 (1):8–12.
7. Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003 May-Jun;15 (3):91–133.
8. Ra SH, Su A, Li X, Zhou J, Cochran AJ, Kulkarni RPet al . Keratoacanthoma and squamous cell carcinoma are distinct from a molecular perspective. Mod Pathol. 2015 Jun;28 (6):799–806.
9. Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss Oet al . RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012 Jan 19;366 (3):207–15.
10. Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud Vet al . Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009 Aug 10;27 (23):e59–61.
11. Smith KJ, Haley H, Hamza S, Skelton HG. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009 Nov;35 (11):1766–70.
12. Graubert TA. A call to action for acute lymphoblastic leukemia. N Engl J Med. 2014 Sep 11;371 (11):1064–6.
Siddhant Thukral, 1 University of Missouri-Kansas City, Kansas City, MO, USA.
Lloyd E. King, 2 Vanderbilt University, Nashville, TN, USA.
David H. Horowitz, 2 Vanderbilt University, Nashville, TN, USA.
About the Author: Siddhant Thukral is currently a second-year medical student of a six-year BA/MD program at the University of Missouri–Kansas City School of Medicine in Kansas City, Missouri, USA.
Correspondence Siddhant Thukral, Address: 5100 Rockhill Rd, Kansas City, MO 64110. Email: stry9@mail.umkc.edu
Cite as: Thukral S, King LE Jr, Horowitz DH. Multiple Keratoacanthomas, Philadelphia Chromosome+ Acute Lymphoblastic Leukemia, and Dasatinib: A Case Report. Int J Med Students. 2015 Sep-Dec;3(3):155-8.
Copyright © 2015 Siddhant Thukral, Lloyd E. King Jr., David H. Horowitz
International Journal of Medical Students, VOLUME 3, NUMBER 3, December 2015