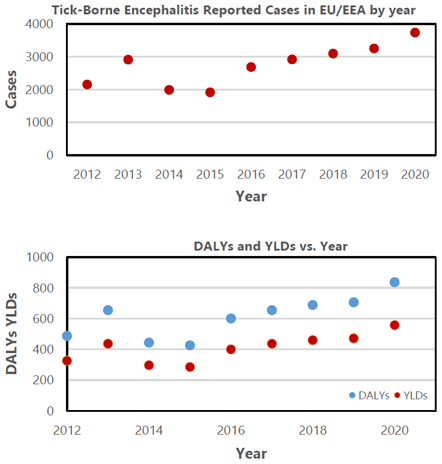
Historical Data Indicating the Number of Reported Cases in EU/EEA (above) and DALY and YLD (below), from 2012 to 2020.
Aswathy Varma1, Marta Szlaszynska1, Assaf Ben-Haim1, Neofytos Ilia1, Silvia Tarricone1, Justyna Lewandowska-Bejm2, Francesco Visentin1, Annalisa Gadler1
doi: http://dx.doi.org/10.5195/ijms.2022.1464
Volume 10, Number 3: 252-257
Received 31 03 2022; Rev-request 23 04 2022; Rev-request 04 06 2022; Rev-request 27 08 2022; Rev-recd 11 05 2022; Rev-recd 22 06 2022; Rev-recd 29 08 2022; Accepted 29 08 2022
ABSTRACT
Background:Tick-borne encephalitis (TBE) is a central nervous system disease that is posing a growing public health challenge in Europe. Despite carrying a significant global impact, its disease burden is still relatively unexplored. This study aims to outline a regression model of how the increasing cases will influence the burden of TBE in the upcoming years, using years lived with disability (YLDs) and disability-adjusted life years (DAYs), and address climate change as a determinant.
Methods:Information regarding the number of cases, and YLDs and DALYs of TBE was collected from European countries using available surveillance data from 2012 to 2020. Number of TBE cases and burden projections were created until 2025, using a linear regression model. The total reported cases of TBE cases in this timeframe, age-group, and gender distribution were inserted and modeled in ECDC BCoDE Toolkit- a software application that calculates the burden of communicable diseases, YLDs and DALYs of each year. A non-systematic bibliographic search was conducted exploring the impact of climate change on TBE.
Results:Our findings showed a linear growth in number of TBE cases (74.3% increase), DALYs (71.3%), and YLDs (71.75%) in European countries from 2012 to 2020. By 2025, these factors are likely to increase by 141% (95% CI: [108%, 175%]), 134% (95% CI: [91%, 177%]) and 134% (95% CI: [98%, 172%]) compared to 2012, respectively (p<0.0001).
Conclusions:The likelihood of morbidity and mortality increase of TBE as well as climate-related changes in tick activity highlight that prompt action is necessary by introducing preventive measures in European populations.
Keywords: Climate Change; Encephalitis, Tick-Borne; Epidemiology; Europe; Global Burden of Disease; Public Health; Surveillance (Source: MeSH-NLM).
Tick-borne encephalitis (TBE) is a central nervous system disease that has been increasingly spreading in Europe from its traditional endemic areas in China, Mongolia, and Russia. It is caused by a virus of the family Flaviviridae and can lead to a wide range of clinical manifestations.1,2 The majority of patients, approximately 70% to 85%, who contract the infection are asymptomatic, which may imply a significant number of undiagnosed cases. However, about 75% of patients infected with the European TBE virus subtype have experienced a biphasic disease course.3 The initial phase is related to viremia during which patients display nonspecific symptoms such as fever, myalgia, arthralgia, fatigue, general malaise and anorexia. This phase lasts for two to seven days followed by amelioration or an asymptomatic interval of about a week. Thereafter, the second phase begins, in which 50% of adults present with meningitis, 40% with meningoencephalitis and 10% with meningoencephalomyelitis. The symptoms of meningitis include the classic triad of high fever, headache, nausea and vomiting, as well as photophobia and vertigo reported by some patients. Encephalitis typically manifests with impaired consciousness, ranging from somnolence to stupor. Meningoencephalomyelitis is usually characterized by flaccid paralysis.3 The virus life-cycle occurs through consistent interactions among intermediate hosts, such as rodents, dears, and ticks-Ixodes ricinus being the most common in Europe and Ixodes persulcatus in the Eastern and Siberian regions.4 The main route of infection in humans is through a tick bite, while contracting the infection via consumption of infected unpasteurised dairy products is less common.5 There is still no specific antiviral therapy to combat TBE. Mainstay options are supportive or, in severe cases, hospitalization in intensive care units with ventilatory support is required. Although many patients with TBE will recover, up to one third will suffer from long-term complications such as nonparalytic encephalitis and chronic TBE.2 Due to limited therapeutic options, preventive measurements such as vaccinations are essential to reduce the devastating outcomes of TBE.
Similarly, the coronavirus disease 2019 (COVID-19) pandemic has revealed several neurological consequences in which symptoms of COVID-19 encephalitis mimic that of TBE.6 Majority of COVID-19 patients are asymptomatic or mildly symptomatic. However, there have been overlaps in neurological manifestations of this infection with TBE in severely ill patients, such as encephalopathies with or without psychosis, encephalitis, acute disseminated encephalomyelitis and myelitis. TBE is a disease that easily preventable with vaccinations. In a similar fashion, the COVID-19 vaccines have significantly decreased morbidity and mortality of the disease, allowing the prevention of these devastating neurological complications.7
Recently, TBE has posed a growing health risk to populations in western European countries that has been associated with climate change-partly because it alters and expands the niche of tick, which is a disease vector.8–12 We hypothesized that the increase in the likelihood of arthropod-borne diseases will consequently lead to a rise in the overall burden of TBE.13
Our study aims to: (I) illustrate the increasing number of TBE cases since 2012; (II) assess the burden of TBE through assessment of Years lived with disability (YLDs) and Disability adjusted life years (DALYs); (III) predict the future trend through a linear regression model; and (IV) evaluate climate change as a driving factor in the increasing burden.
We used the online Surveillance Atlas of Infectious Diseases of the European Centre for Disease Prevention and Control (ECDC) to obtain number of TBE cases from 2012 to 2020 in Europe.14 The Surveillance Atlas of Infectious Diseases is a tool that presents the latest data obtained through The European Surveillance System (TESSy) from the Member States on several infectious diseases. The total reported cases (2012-2020), age-group, and gender distribution were then inserted and modeled in the ECDC BCoDE Toolkit, a software application that allows the calculation of the burden of disease of a variety of communicable diseases, to calculate YLDs and DALYs of each year.15 DALYs and YLDs allow for a measure of disease burden and injury in a population. One DALY represents the loss of the equivalent of one year of full health and one YLD represents the equivalent of one healthy year lost due to disability.16 Of note, when data collection at the EU level was initiated, twenty countries reported their TBE cases, whereas by the year 2020, reported cases from five more countries were included. Our data analysis did not include these additional reports as the Paired Wilcoxon Signed-Rank test deemed these additions insignificant.
On both raw data plots (Figure 1), we observed that the number of TBE cases, YLDs, and DALYs follow the increasing trend from 2014. Therefore, we decided to use observations from 2014 to estimate the maximum likelihood of each variable. A linear regression model was created for each dataset in RStudio, and we used metrics such as Residual Standard Error, R-squared, t-statistics and the F-statistics to confirm that the models fit the data. Residual Standard Error and F-statistics were used to measure how well the regression model fits the dataset. Linear regression was used because it was the simplest model for seven data points. Moreover, we utilized R-Squared that indicates the proportion of variation in the dependent variable as explained by the model. Finally, we used t-statistics to test for the significance of the “year” variable in each model. To assess the overall model significance, a two-sided p-value was obtained with a cut-off set up to 0.05 (as outlined in Table 1).
Historical Data Indicating the Number of Reported Cases in EU/EEA (above) and DALY and YLD (below), from 2012 to 2020.

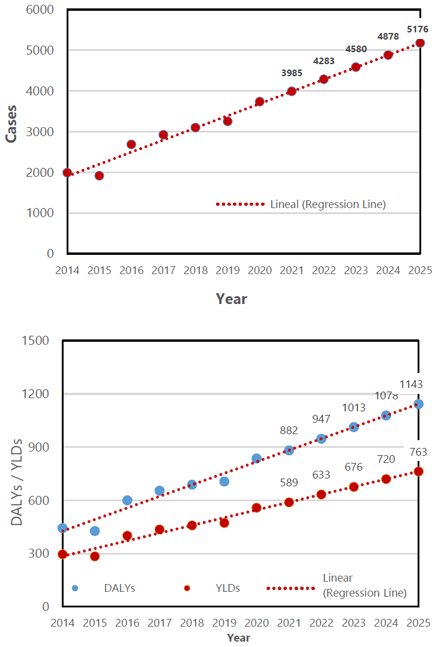
Regression Models Characteristics, Demonstrating p-value and Residual Standard Error and Estimated Values for Number of New TBE Cases, YLDs and DALYs from 2021 to 2025.
| Year\Linear regression model | TBE New Cases vs. Year p-value [F-test] <0.0001 RSE = 180.50 |
YLDs vs. Year p-value [F-test] <0.0001 RSE = 29.80 |
DALYs vs. Year p-value [F-test] <0.0001 RSE = 44.42 |
|---|---|---|---|
| 2021 | 3985 (95% CI: 3593–4377) |
589 (95% CI: 524–654) |
882 (95% CI: 733–1032) |
| 2022 | 4283 (95% Cl: 3811–4755) |
633 (95% Cl: 555–710) |
947 (95% Cl: 785–1110) |
| 2023 | 4580 (95% Cl: 4026–5135) |
676 (95% Cl: 585–768) |
1013 (95% Cl: 835–1191) |
| 2024 | 4878 (95% Cl: 4240–5517) |
720 (95% Cl: 614–825) |
1078 (95% Cl: 883–1272) |
| 2025 | 5176 (95% Cl: 4453–5899) |
763 (95% Cl: 644–883) |
1143 (95% Cl: 931–1354) |
To understand the possible effects of environmental alterations on the life cycle of tick species and on the rising number of TBE cases, we performed a structured, non-systematic bibliography search of the PubMed database. We included reviews, laboratory, observational and modeling studies that focused primarily on the link between climate change and changes in tick and TBE distribution in Europe. Case reports, case series and surveys were excluded. The search strategy included (((ticks) OR (TBE)) OR (tick-borne encephalitis)) AND ((climate change) OR (global warming)). 413 articles were identified, from which only those in English were included. No publication date restrictions were applied, and articles describing solely other tick-borne diseases were not considered. The first search was performed on the 14th of October 2021 and updated thereafter. The literature screening and the selection was carried out independently by three reviewers. Additionally, well-structured reviews on TBE and/or ticks and climate change were consulted, and its cross-references were screened. Altogether, three reviewers identified 10 articles that were most relevant to our analysis.
While in 2012 there were 2142 reported cases of TBE in Europe, there were 3734 cases in 2020, indicating a 74.3% rise in eight years. Regarding the disease burden in the EU from 2012 to 2020, DALYs increased from 488 to 836 (71.3% increase) with a similar increase in YLDs from 325 to 558 (71.7% increase).
The raw data suggest a linear relationship between consecutive years and all three variables (Figure 1). The value of the R-squared coefficient in the three models is above 0.9, indicating that the variability of the response variables is well explained by the year. The R-squared coefficient of TBE reported cases model from 2012-2020 is 0.9384 (95% CI: 0.8784058, 0.9911942). The R squared value for YLDs versus year model is 0.9228 (95% CI: 0.8564565, 0.9891435) and DALYs versus year model is 0.9232 (95% CI: 0.8571859 0.9892141).
The regression model created from the estimated number of new cases revealed 3985 cases in the year 2021, and a rise to 5176 cases in 2025 (95% CI: 4453-5899). The DALYs was predicted to increase from 882 in 2021 to 1143 in 2025 (95% CI: 931-1354). Finally, the predicted YLD value was found to be 589 in 2021, and 763 in 2025 (95% CI: 644-883). The estimated values of new cases, DALYs and YLDs in 2025 corresponded to a 141% (95% CI: [108%, 175%]), 134% (95% CI: [91%, 177%]) and 134% (95% CI: [98%, 172%]) increase from those in 2012, respectively. The confidence intervals and values calculated for each predicted year is outlined in Table 1 and Figure 2.
Tendency Line Demonstrating Likelihood Increase in New Cases from 2021 to 2025 (above) and Predicted Rates of DALYs and YLDs from 2021 to 2025 (below).
Since the initiation of the data registry amongst the EU/EFTA countries in 2012, the number of TBE cases showed an overall major increase of 74.3% by 2020, based on the latest available information.14 This was accompanied by an increased disease burden quantified by DALYs and YLDs. Based on our projections, a 175% increase in TBE cases from 2012 is possible by the year 2025, hence highlighting the need for prompt action. A contributing factor to the expansion of TBE is the continuous spread of ticks’ species, largely associated with climate variability.
Climate change has been suggested to have a direct influence on the epidemiology of vector-borne disease.13 A predicted increase of 1.0 – 3.5 degrees Celsius is estimated by the year 2100, potentially causing an increase in the likelihood of many vector-borne diseases. TBE is not an exception and is becoming a public health concern.8,13 According to former studies, the changes in the density and distribution of the tick population, particularly the shift to previously hostile areas and the associated increase in tick-borne pathogen transmission, are influenced by climate variability, most notably rising temperatures.10,11
Environmental changes are thought to be a selective pressure inducing the adaptation in both ticks’ physiology and behavior. Laboratory and field studies have elucidated the biological mechanism that potentially underlies this alteration in density and distribution as ticks are susceptible to temperature and humidity.17,18 It was shown that rising temperature causes acceleration of the cycle development and the production of eggs. Furthermore, the incubation period and stages of the tick life cycle have a shorter duration when exposed to higher temperatures.18,19 Altogether, these could boost transmission risk through increased vector population within a favorable temperature range and as a result, an increased TBE morbidity in the EU/EFTA can be expected.
Correspondingly, models that use satellite data to represent a change in environmental factors important for sustaining foci of TBE, suggest that climate change could be partly responsible for increasing number of cases in Europe.12,20,21 Indeed, Species Distribution Modeling of the climatic niche of the most important vector of TBE in Eurasia (tick Ixodes ricinus) shows an increase of a climatically suitable area between 2050 and 2080 of about two times greater than the current area.20 Moreover, a rise in temperature and a decrease in moisture in the summer is likely to cause a shift in distribution of TBE into higher-latitude and higher-altitude regions progressively through the 2020s, 2050s, and 2080s.21
The shift estimated by the models is supported by already observed northern longitudinal extension of ticks in Sweden and expansion of the ticks population into higher altitudes in Central Europe in Czech Republic.10,11 Both examples demonstrate an establishment of the tick population in the new, previously hostile area for their survival and development.
However, it is important to take into consideration that estimating the impact of global warming quantitatively on the epidemiology of TBE is difficult, due to the complex interplay between biotic and abiotic factors that influence hosts, arthropods and pathogens. Additionally, climate-related changes in human behavior must be considered, such as more time spent outdoors due to increased number of warmer days, which contributes to the rising number of TBE cases by putting people directly at a risk of infection.9,22
Our study has some limitations. For instance, it included combined data that were collected from individual countries in the EU/EFTA, which have different reporting systems and preventive measures. In addition, ECDC has reported under-ascertained infections and under-reporting across countries, thereby limiting the number of reported cases used in our study. Data collected are limited to the years 2012-2020 as ECDC initiated EU surveillance of TBE cases in 2012. Having a larger dataset with a unified data-collecting protocol would enable us to confirm with better accuracy that the linear trend was chosen correctly. Therefore, the projected increasing trend may not be representative of a longer period of time. Most importantly, the five-year estimations are solely based on yearly statistical data that was collected without consideration of any other variables. Moreover, we conducted nonsystematic bibliographic search when exploring climate change as a plausible driving factor in increasing TBE burden. Due to these confounding factors, we suggest that future TBE-related projections be studied in countries at risk and more systematic approach to be undertaken when examining causative factors.
Our study explored the increasing number of TBE cases in Europe since 2012, in relation to the expansion of endemic areas and the prolongation of tick activity season, resulting in an increase in the disease burden. We offered a simplified model, based on limited data, which showed the likely continuation of this trend in the coming years, with the aim to increase countries’ awareness of this largely preventable disease. At present, vaccination policies vary with nations, and low vaccine coverage for populations at risk is a definite factor for increase morbidity. In Austria, for instance, mass vaccination campaign that began in 1981 and reached a coverage of 82% for its entire population by 2015 dramatically reduced annual cases compared to pre-vaccination era, whereby Austria held the highest recorded TBE-related morbidity in Europe.23 By contrast, the Czech Republic, with a similar surveillance of TBE, only 10% of its at-risk population was vaccinated by the year 2000, and an opposite trend was observed when cases continued to rise each year, highlighting the effectiveness of an extensive TBE vaccine coverage. In addition, in some countries the risk of TBE is underestimated, likely due to low awareness among physicians.24
Amongst preventive measures, individuals partaking in outdoor activities should be informed that long sleeve clothing, insect repellents, and prompt eventual tick removal, are effective ways to avoid TBE infection. Moreover, strategies for effective locally adapted sustainable vector control, as indicated in the WHO document Global Vector Control Response 2017-2030, are essential to diminish TBE impact. Apart from raising public awareness regarding the risks of tick bites, vaccinations in endemic areas remain the most crucial and powerful preventive tool available for protection against TBE, as previously mentioned.25 WHO recommends to establish public immunization strategies and provide vaccinations to all groups in areas where the disease is highly endemic, defined as an incidence of clinical disease of ≥ 5 cases/per 100,000 population per year.26 Since definitive therapeutic options are not yet available, it is of key importance that primary prevention measures are implemented to control transmission and infection, especially in the regions and countries that have experienced the largest increase in TBE cases.
We thank Mario Raviglione, Professor of Global Health in University of Milan, researcher, and Director of the WHO Global TB Program from 2013-2017, for his expertise and assistance throughout all aspects of our study and for his help in editing this manuscript
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: MS, AG. Data Curation: ABH. Formal Analysis: JBL. Investigation: AHB, ST, AG. Methodology: MS, ABH, AG. Project Administration: AV. Resources: MS, NI, JLB. Supervision: AV, MS. Validation: MS, AG. Writing – Draft Preparation: AV, MS, FV, AG. Writing – Review & Editing: AV, MS, AG.
1. Kaiser R. [Tick-borne encephalitis]. Nervenarzt. 2016;87(6):667–80.
2. Riccardi N, Antonello RM, Luzzati R, Zajkowska J, Di Bella S, Giacobbe DR. Tick-borne encephalitis in Europe: a brief update on epidemiology, diagnosis, prevention, and treatment. Eur J Intern Med. 2019;62:1-6.
3. Bogovic P, Strle F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J Clin Cases. 2015;3(5):430–41.
4. Michelitsch A, Wernike K, Klaus C, Dobler G, Beer M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses. 2019;11(7).
5. Buczek AM, Buczek W, Buczek A, Wysokinska-Miszczuk J. Food-Borne Transmission of Tick-Borne Encephalitis Virus-Spread, Consequences, and Prophylaxis. Int J Environ Res Public Health. 2022;19(3).
6. Peterson CJ, Sarangi A, Bangash F. Neurological sequelae of COVID-19: a review. Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):122.
7. Marsh EB, Kornberg M, Kessler K, Haq I, Patel AD, Nath A, et al. COVID-19 and Vaccination in the Setting of Neurologic Disease: An Emerging Issue in Neurology. Neurology. 2021.
8. Randolph SE. Evidence that climate change has caused 'emergence' of tick-borne diseases in Europe? Int J Med Microbiol. 2004;293 Suppl. 37:5–15.
9. Randolph SE. The shifting landscape of tick-borne zoonoses: tick-borne encephalitis and Lyme borreliosis in Europe. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):1045–56.
10. Daniel M, Materna J, Honig V, Metelka L, Danielova V, Harcarik J, et al. Vertical distribution of the tick Ixodes ricinus and tick-borne pathogens in the northern Moravian mountains correlated with climate warming (Jeseniky Mts., Czech Republic). Cent Eur J Public Health. Sep. 2009;17(3):139–45.
11. Jaenson TG, Jaenson DG, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit Vectors. 2012;5:8.
12. Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip Perspect Infect Dis. 2009;2009:593232.
13. Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78(9):1136–47.
14. European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases; Available from: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases. Last updated May 31, 2017; cited Oct 17, 2021.
15. European Centre for Disease Prevention and Control. Toolkit - application to calculate DALYs; Available from: https://www.ecdc.europa.eu/en/publications-data/toolkit-application-calculate-dalys. Last updated March 21, 2019; cited October 20, 2020.
16. Cao B SG, Ho J, Ma D. World Health Organisation. WHO methods and data sources for global burden of disease estimates 2000-2019.; Available from: https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2019_daly-methods.pdf?sfvrsn=31b25009_7. Last updated December, 2020; cited Nov 02, 2021.
17. Gray JS. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. International Journal of Medical Microbiology. 2008;298:19–24.
18. Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O'Callaghan CJ, et al. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J Med Entomol. 2004;41(4):622–33.
19. Rossati A. Global Warming and Its Health Impact. Int J Occup Environ Med. 2017;8(1):7–20.
20. Porretta D, Mastrantonio V, Amendolia S, Gaiarsa S, Epis S, Genchi C, et al. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasit Vectors. 2013;6:271.
21. Randolph SE, Rogers DJ. Fragile transmission cycles of tick-borne encephalitis virus may be disrupted by predicted climate change. Proc Biol Sci. 2000;267(1454):1741–4.
22. Esteve-Gassent MD, Castro-Arellano I, Feria-Arroyo TP, Patino R, Li AY, Medina RF, et al. Translating Ecology, Physiology, Biochemistry, and Population Genetics Research to Meet the Challenge of Tick and Tick-Borne Diseases in North America. Arch Insect Biochem Physiol. 2016;92(1):38–64.
23. Kunz C. TBE vaccination and the Austrian experience. Vaccine. 2003;21 Suppl 1:S50–5.
24. Mohareb E, Christova I, Soliman A, Younan R, Kantardjiev T. Tick-borne encephalitis in Bulgaria, 2009 to 2012. Euro Surveill. 2013;18(46).
25. Zavadska D, Anca I, Andre F, Bakir M, Chlibek R, Cizman M, et al. Recommendations for tick-borne encephalitis vaccination from the Central European Vaccination Awareness Group (CEVAG). Hum Vaccin Immunother. 2013;9(2):362–74.
26. Taba P, Schmutzhard E, Forsberg P, Lutsar I, Ljostad U, Mygland A, et al. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol. 2017;24(10):1214–e61.
Aswathy Varma, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
Marta Szlaszynska, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
Assaf Ben-Haim, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
Neofytos Ilia, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
Silvia Tarricone, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
Justyna Lewandowska-Bejm, 2 Second semester, Master's Program, Faculty of Mathematics and Information of Science, Warsaw University of Technology, Warsaw, Poland.
Francesco Visentin, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
Annalisa Gadler, 1 Sixth-year Medical Student. International Medical School, University of Milan, Milan, Italy.
About the Author: Aswathy Varma is currently a sixth-year medical student of University of Milan, Milan, Italy of a six-year program. She was the first author and presented an abstract to Asia Pacific AIDS & Co-infections Conference (APACC) 2020.
Correspondence: Aswathy Varma. Address: Via Festa del Perdono, 7, 20122 Milano MI, Italy. Email: avarma0702@gmail.com
Editor: Adnan Mujanovic Student Editors: André Yvan Zolo & Patricio García-Espinosa Layout Editor: Ana Maria Morales Process: Peer-reviewed
Cite as: Varma A, Szlaszynska M, Ben-Haim A, Ilia N, Tarricone S, Lewandowska-Bejm J, Visentin F, Gadler A. Bearing the Burden of Tick-Borne Encephalitis in Europe, 2012-2020: Rising Cases, Future Predictions and Climate Change. Int J Med Stud. 2022 Jul-Sep;10(3):252-7.
Copyright © 2022 Aswathy Varma, Marta Szlaszynska, Assaf Ben-Haim, Neofytos Ilia, Silvia Tarricone, Justyna Lewandowska-Bejm, Francesco Visentin, Annalisa Gadler
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 3, September 2022