Original Article
Prognostic Factors of Survival in Veno-Arterial ECMO Patients: A Multivariable Logistic
Regression Analysis
Andrew Jones1, George Olverson2, Wayne Wong3, Rohun Bhagat4, Clauden Louis5
doi: http://dx.doi.org/10.5195/ijms.2023.1557
Volume 11, Number 4: 285-293
Received 05 06 2022;
Rev-request 08 05 2022;
Rev-recd 02 04 2022;
Accepted 29 11 2023
ABSTRACT
Background:
Several models exist to predict mortality in patients on Veno-arterial (VA) extracorporeal
membrane oxygenation (ECMO). Whether expanded demographic data points have prognostic
implications is less understood. This study assessed the prognostic value of demographics
in patients on VA-ECMO.
Methods:
This retrospective cohort study investigated 410 patients who received VA-ECMO. Survival
to hospital discharge, survival to intensive care unit discharge, and survival to
ECMO explantation were examined. A multivariable logistic regression was performed
incorporating 11 demographic variables.
Results:
44% (181/410) of patients survived to ECMO explant, 37% (152/410) of patients survived
to ICU discharge, and 36% (146/410) of patients survived to hospital discharge. There
was a statistically significant increase in odds of survival to hospital discharge
in older patients. Within the age range of the study population, for each additional
year of age there was a 1% increase in odds of survival. There was a decrease in odds
of survival to hospital discharge in patients who had a prior cardiac arrest (OR =
0.82 p = 0.0003). Patients who survived to hospital discharge less frequently had
a history of dialysis (OR = 0.81, p = 0.0348).
Conclusion:
Older age was a prognostic indicator of survival to hospital discharge following VA-ECMO,
while a history of dialysis and history of cardiac arrest were associated with mortality.
Sex, BMI, atrial fibrillation, hypertension, DM, and COPD were not significant indicators.
These data may help guide optimal patient selection for VA-ECMO support.
Introduction
ECMO is used as a temporary adjunct for respiratory and cardiac support in patients
with either severe respiratory failure or cardiogenic shock.1 Featuring large bore cannulas, an external oxygenator, a temperature control unit,
and a pump circuit, ECMO has been used increasingly in intensive care unit settings
for patients refractory to conventional therapeutics. This highly invasive procedure
requires substantial training in the initiation and maintenance of ECMO physiology.
Veno-Venous ECMO (VV-ECMO) continues to be used for patients in respiratory failure
with preserved cardiac function,2 treating acute respiratory distress syndrome (ARDS) patients, where it has been instrumental
in providing lung rest, while Veno-Arterial ECMO (VA-ECMO) has allowed for both cardiac
rest and end organ resuscitation.
As ECMO has grown in prevalence due to its ability providing to support patients until
more definitive, durable cardiac recovery can be achieved.3 The prognostic implications of this increase in prevalence, however, hinge on a multitude
of factors, especially as higher risk cohorts with additional comorbidities are provided
ECMO support.4 Both the ethical concerns of poor ECMO candidate selection, and the resource requirements
make identifying optimal candidates for ECMO a critical, and practical part of any
successful ECMO program. Giving clinicians tools to predict who will be successfully
bridged to recovery is of paramount importance. Several studies have described prognostic
factors associated with VV or VA-ECMO, but due to the differences in indications,
optimal candidates for VV or VA-ECMO differ significantly.
The COVID-19 pandemic has spurred research in selecting candidates for VV-ECMO,5-7 with 2020 yielding more ECMO research than any year prior, but questions remain about
the optimal VA-ECMO candidate.8 The Survival After Veno-Arterial ECMO (SAVE) score,9, 10 duration of ECMO support,11 and other lab values have been used to describe the prognosis of candidates for VV-ECMO,
and VA-ECMO, but additional demographic, comorbidities, and disease factors are not
well understood or described.12,13 Identifying these traits to help better identify optimal candidates for limited availability14 going forward is of central importance to ensuring positive patient outcomes, safe
staffing ratios,15,16 and managing goals of care. This study seeks to help bridge that gap.
Methods
We performed a retrospective cohort analysis of a major heart failure center for patients
who received veno-arterial extracorporeal membrane oxygenation (VA-ECMO) between 2016-2020.
We identified 545 patients over the age of 18 who underwent all categories of ECMO.
122 patients were excluded because they underwent veno-venous ECMO, while an additional
13 were excluded for receiving a configuration of ECMO which was not considered to
be purely veno-arterial throughout their ECMO duration (e.g., VA-ECMO to right ventricular
assist device or mixed Veno-arterial-venous ECMO). This study was approved by University
of Rochester's RSRB (ID: STUDY00007291).
We utilized retrospective electronic medical record chart review in conjunction with
data collected through the University of Rochester Medical Center (URMC) ECMO QA/QI
database previously validated in prior research17,18 to build a dataset including demographic, clinical, and outcome data for this patient
population. Specifically, age, sex, body mass index (BMI), history of hypertension,
history of diabetes mellitus (DM), history of chronic obstructive pulmonary disease
(COPD), history of atrial fibrillation (AF), history of smoking, history of dialysis,
origin of cardiomyopathy (ischemic vs nonischemic vs mixed) and history of prior cardiac
arrest were collected by a trained data abstraction team from EMR. The authors standardized
training between abstractors to ensure homogeneous data definitions, and criteria,
but abstractors were not blinded to the hypothesis.
The primary outcome of interest was survival to discharge from the hospital. Secondary
outcomes included ECMO explantation and discharge from the intensive care unit (ICU).
Explantation was defined as removal of ECMO without replacement for greater than 24
hours. Discharge from the ICU was defined as removal of ECMO with stable hemodynamics
(further defined as not requiring vasoactive chemotherapeutics) and otherwise meeting
clinical criteria for floor status. Discharged from the hospital was defined as discharge
from the floor with placement being either home, physical medicine rehabilitation
(PM&R) center, or skilled nursing facility (SNF).
A multivariable logistic regression was also performed to analyze outcomes at three
different clinical endpoints (explantation of ECMO support, ICU discharge, or hospital
discharge) incorporating 11 variables selected by the authors as likely to have a
direct physiologic impact on prognosis. Variables were selected based on previously
validated models such as the SAVE score.19 Additional variables were selected based on biologic plausibility; variables that
do not have a clear biological mechanism were excluded. For completeness of prognostic
information, all patients were included in all phases of analysis. Patients who died
prior to explantation were also counted in those who died prior to ICU discharge.
Each clinical endpoint was analyzed separately, preventing censuring, or competing
risk.
After performing multivariable logistic regression, we performed bidirectional stepwise
selection of models. The final models including the statistically significant prognostic
values are included in Table 3.
For univariate analysis, Shapiro-Wilkes test was used to test for normalcy. Normal
variables were tested with a Welch two-sample two-sided t-test. Variables found to
be non-normal were tested using a Mann-Whitney U test. An additional Welch two-sample
two-sided t-test or chi squared test was performed for variables that arose during
the patient's ECMO course such as time on VA-ECMO support and location of ECMO cannulas.
As these are not factors present prior to cannulation, these variables were not included
in the multivariable logistic regression analysis. A significance threshold of 0.05
was chosen. The C statistic of the model generated was measured to compare to the
C statistic of the SAVE score. R Studio Software (Version 1.4.1717) was utilized for
data analysis. Google Documents and Microsoft Word were used for generating tables
and figures.
Results
Of 410 patients who were included in the study, the mean age was 55.2 years old (range:
19 years to 90 years). 32% of patients were female. The average BMI was 31 kg/m2.
26% (107) of patients had a history of atrial fibrillation, 63% (260) has a history
of hypertension, 33% (134) had a history of diabetes mellitus, 15% (62) had a history
of COPD, 60% (247) had a history of smoking, 6% (24) had a history of dialysis, and
23% (96) had a history of cardiac arrest. Demographics and descriptive characteristics
were also stratified by survival to hospital discharge. Complete demographic characteristics
are found in Table 2.
Of 410 patients, 44% (181/410) of patients survived to ECMO explant. 37% (152/410)
of patients survived to ICU discharge. 36% (146/410) of patients survived to hospital
discharge. (Figure 1) For the following analyses, findings reaching significance are described textually
while complete findings (significant and non-significant) are reported in Tables 3-4.
Figure 1
ECMO Course Flowsheet.
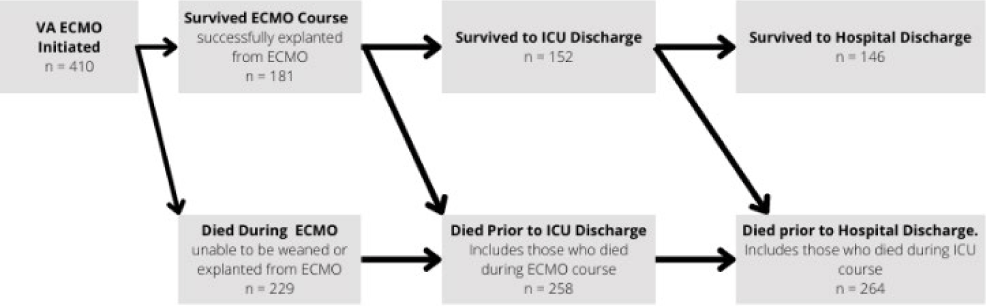
Legend: for completeness of prognostic information, all patients were included in all phases
of analysis. Patients who died prior to explantation, were also counted in those who
died prior to ICU discharge. Each clinical endpoint was analyzed separately, preventing
censuring, or competing risk.
After the stepwise model was built predicting survival to ECMO explant, the following
variables remained: prior cardiac arrest, and age. There was a slight decrease in
odds of survival to explantation in patients who were younger (Odds Ratio (OR) = 0.99,
p<0.0001). There was a decrease in odds of survival to explantation in patients who
had a prior cardiac arrest (OR = 0.77, p <0.0001). A t-test or chi-squared test was
performed to further characterize associations between survival to ECMO explantation
(for continuous variables and categorical variables respectively) (age and prior cardiac
arrest). Of patients who survived to explantation, the mean age was 53.3+/-14.8 years;
of patients who did not survive to explantation, the mean was 56.8+/-16.6 (p = 0.030).
Patients who survived to explantation were less likely to have a history of cardiac
arrest when compared to patients who did not survive to explantation (13% (23) v.32%
(73), respectively; p <0.0001).
An additional stepwise multivariable logistic regression was run to investigate survival
to ICU discharge. There was a decrease in odds of survival to ICU discharge in patients
who were younger (OR = 0.99, p <0.0001). There was a decrease in odds of survival
to ICU discharge in patients who had a prior cardiac arrest (OR = 0.35 p = 0.0002).
A t-test or chi-squared test was performed to further characterize associations between
survival to ECMO explantation (for continuous variables and categorical variables
respectively). Of patients who survived to ICU discharge, their average age was 51.6
+/- 14.2 compared to an average age of 57.4+/- 16.5 in folks who did not survive to
ICU discharge (p = 0.0002). Patients who survived to ICU discharge were less likely
to be receiving dialysis (4% (6) v. 7% (18)), but this association was only present
in multivariate analysis (univariate p = 0.18). Similar to ECMO explant, patients
who survived to ICU discharge were less likely to have a history of cardiac arrest
compared to patients who did not survive to ICU discharge (13% (20) v. 29%, (76) respectively;
p <0.0001).
A third stepwise multivariable logistic regression was examining survival to hospital
discharge. There was an increase in odds of survival to hospital discharge in patients
who were older (OR = 0.99, p<0.0001). There was a decrease in odds of survival to
hospital discharge in patients who had a prior cardiac arrest (OR = 0.82, p = 0.0003.
There was also a decreased odds of survival to hospital discharge in patients with
a history of hemodialysis (OR = 0.81, p = 0.0348). Of patients who survived to hospital
discharge, their average age was 51.4+/-14.0 compared to those who did not survive,
with an average age of 57.3+/-16.5 (p = 0.002). Rates of dialysis were lower among
those who survived to hospital discharge as well (7% (19 v. 3% (5)), but this association
is only significant in the multivariate model (p = 0.107). Similarly, patients who
survived to hospital discharge had lower rates of cardiac arrest compared to patients
who did not survive to hospital discharge (13% (19) v. 29% (77), respectively; p =
0.0002). No other statistically significant associations were noted. Of note, the
SAVE score is quoted as having a C statistic of 0.68, while the calculated C statistic
of this new model is 0.708.
When comparing time on VA-ECMO support by all outcome variables, there were no correlations
found between length of VA-ECMO run time and outcome. Specifically, those explanted
had a similar time receiving ECMO support to those who were not explanted (205 v.
174 hours, respectively; p=0.09). Additionally, those who were discharged from the
ICU had a similar time receiving ECMO support to those who were not discharged from
the ICU (191 v. 185 hours, respectively; p=0.77). Lastly, those who were discharged
from the hospital had a similar run time to those who were not discharged from the
hospital (182 v. 191 hours, respectively; p=0.65).
Utilizing findings from both the multivariable logistic regressions and t-tests, we
summarized the protective prognostic factors versus the harmful prognostic factors
of discharge from the hospital following VA-ECMO (Table 1). This study found older age to be protective in predicting discharge from the hospital
following VA-ECMO. History of smoking, dialysis, or cardiac arrest were found to be
harmful in predicting discharge from the hospital following VA-ECMO.
Table 1
Protective Prognostic Factors v. Harmful Prognostic Factors in Discharge from Hospital.
| Protective Factors |
Harmful Factors |
- Older age
- No history of dialysis
- No history of cardiac arrest
|
- Younger age
- History of dialysis
- History of cardiac arrest
|
Table 2
Demographics and Descriptive Characteristics of Patient Cohort.
|
All Patients (n=410) |
Survived to Hospital Discharge (n = 146) |
Died in Hospital (n = 264) |
p-Value |
| Age at Hospitalization (Mean; Years Old) +/- std dev [min, max] |
55.2 +/- 15.93
[19, 90] |
51.4 +/- 14.03
[19, 79] |
57.3 +/- 16.54
[19, 90] |
< 0.0001* # |
| Female (%) |
31.7 |
30.1 |
32.6 |
0.61 |
| BMI (kg/m2)
|
30.9 +/- 7.25 |
30.6 +/- 6.74 |
31.0 +/- 7.53 |
0.78 # |
| Atrial Fibrillation (%) |
26.1 |
24.0 |
27.3 |
0.53 |
| Hypertension (%) |
63.4 |
62.3 |
64.0 |
0.73 |
| Diabetes Mellitus (%) |
32.7 |
31.5 |
33.3 |
0.71 |
| COPD (%) |
15.1 |
11.6 |
17.1 |
0.14 |
| Smoking (%) |
60.2 |
51.4 |
65.2 |
0.006* |
| Dialysis (%) |
5.9 |
3.4 |
7.2 |
0.12 |
| Cardiac Arrest (%) |
23.4 |
13.0 |
29.2 |
0.0002* |
| Non-Ischemic Cardiomyopathy (%) |
43.92% |
56.25% |
37.07% |
0.001* |
Legend:
*Indicates statistical significance of p-value.
#indicates continuous variables that were determined to be non-normally distributed
and thus tested with a Mann-Whitney U test instead of a Welch two-sample two-sided
t-test.
Table 3
Multivariable Logistic Regressions of Survival to Various Endpoints.
| Characteristic |
ECMO Explant |
ICU Discharge |
Hospital Discharge |
|
OR (95 CI) |
p value |
OR (95 CI) |
p value |
OR (95 CI) |
p value |
| Younger Age |
0.99 (0.989-0.996) |
<0.0001 |
0.99 (0.988-0.994) |
<0.0001 |
0.99 (0.987-0.994 |
<0.0001 |
| Female |
Not Significant |
|
Not Significant |
|
Not Significant |
|
| BMI |
Not Significant |
|
Not Significant |
|
Not Significant |
|
| Atrial Fibrillation |
Not Significant |
|
Not Significant |
|
Not Significant |
|
| Hypertension |
1.11 (1.00-1.24) |
0.05 |
0.90 (0.998-1.228 |
0.06 |
1.11 (0.998-1.23) |
0.06 |
| Diabetes Mellitus |
Not Significant |
|
Not Significant |
|
Not Significant |
|
| COPD |
Not Significant |
|
Not Significant |
|
Not Significant |
|
| Smoking |
Not Significant |
|
0.93 (0.85-1.02) |
0.14 |
0.93 (0.85-1.02) |
0.11 |
| Dialysis |
Not Significant |
|
0.84 (0.69-1.01) |
0.07 |
0.82 (0.68-0.98) |
0.03 |
| Cardiac Arrest |
0.77 (0.69-0.86) |
<0.0001 |
0.82 (0.73-0.91) |
0.0002 |
0.82 (0.74-0.91) |
0.0003 |
| Ischemic vs Nonischemic Cardiomyopathy |
Not Significant |
|
Not Significant |
|
Not Significant |
|
Legend: Bold indicates significance. 95 CI = 95% Confidence Interval. p values represent
the p value associated with the odds ratio of the associated variable. “Not significant”
values are values that were not selected in the bidirectional stepwise selection process.
The location of ECMO cannula was also investigated. Central ECMO placement (in the
thorax, rather than peripherally in femoral/axillary arteries) was associated with
increased survival to hospital discharge (79.7% (51) v. 61.6% (213); p = 0.005), with
a lower rate of cardiac arrest noted (12.5% (8) in central cohort, 25.43% (88) in
peripheral cohort; p = 0.024) in those receiving central ECMO. Conversely, central
ECMO was associated with no difference in duration of ventilator support, (12.94 v.
13.70 days; p = 0.715), or duration of ECMO support (193.66 hours peripheral v. 154.31hours
central; p = 0.137). There was a difference in rates of cardiomyotomy between central
and peripheral cohorts (p <0.0001), but of note cardiomyotomy was not associated with
a differential in survival to hospital discharge (p = 0.051).
Discussion
Advancing the predictive power of ECMO prognostic models continues to be important
for critical care clinicians. Since ECMO is designed only for short-term intervention,
appropriate allocation of resources is necessary as institutions seek to bridge patients
capable of recovering cardiac function to recovery, destination, or transplant for
definitive support. Given limited resources now more than ever during the time of
pandemics, ethical discussions have led to the need for clarification regarding patient
selection for ECMO.5 Prolonged use of VA-ECMO causes significant hemolysis, inflammation, and other adverse
complications.20,21 Because of this, patients who have a low likelihood of a good outcome, and little
chance of recovery should be considered poor candidates for this technology, further
highlighting the importance of accurate prognostic information as part of ECMO candidate
selection processes.
Published data on pre-ECMO risk factors have aided in the creation of Survival After
Veno-Arterial ECMO (SAVE), a risk prediction model of mortality for patients requiring
ECMO.9 This clinical tool is limited to the specific risk factors included in the study
and shows an association between these variables with mortality. Specifically, history
of smoking, dialysis status, BMI, atrial fibrillation, hypertension, diabetes mellitus,
and COPD were not explored in the study; factors we believe may provide additional
cohort prognostication. Studies allude to the SAVE score underestimating the probability
of survival, while showing no clear trend of survival between the different risk groups
classified within SAVE.19 This research supports that idea, with a slightly larger C statistic (0.708 vs 0.68)
with fewer variables of interest, suggesting that additional prognostic markers may
be fruitful in improving this further.9 Thus, further research is needed to discern additional demographics to provide better
prognosis of ECMO patients. This study seeks to add to the SAVE score, and help clinicians
choose optimal ECMO candidates.
While some might question these findings, with an OR of 0.99 for age, it is important
to remember that this represents an OR of 0.99 for each year older. With the large
age range captured in this study. By nature, age should not have a large magnitude
effect per year, and we see that in the modest, but statistically significant OR consistently
present in all models. Of note: in calculating the OR between the minimum age in this
study (19 years old) and the maximum years of age (90 years old), based on this model,
there is an OR of 4.73.
This study is not without limitations. This study is limited by its retrospective
nature at a single center. This also opens the potential for specific provider bias,
as there are only a few providers who initiate ECMO cannulation at this institution.
Future studies should seek to replicate these findings in a prospective design across
multiple centers and obtain provider information for each patient. Logistic regression
is a robust statistical method, but other studies should seek to integrate these findings
into existing prognostic tools mentioned above to further isolate the impact of the
significant predictors mentioned here. These data did not find a correlation between
the duration of VA-ECMO and survival, in contrast to other studies findings, suggesting
that further investigation is needed to identify the differences in ECMO use across
different centers. While the indications for ECMO can be clear, provider judgment
still plays an important role in candidate selection, further emphasizing the importance
of multi-center trials, where differences at particular centers can be identified.
Similarly, as these data were collected from a wide range of dates, there is a risk
of variable clinical practice over time influencing these results, particularly during
the COVID-19 pandemic.
This study shows older age, no history of dialysis, and no history of cardiac arrest
as protective prognostic factors leading to discharge from the hospital. In comparison,
older age, history of dialysis, and history of cardiac arrest were identified as harmful
prognostic factors (Table 2). The univariate difference between ischemic and non-ischemic cardiomyopathy outcomes
did not retain significance when controlling for other factors, highlighting the importance
of robust, clinically oriented patient considerations during candidate selection.
While the positive association between age and mortality is surprising, the effect
size is small and may not be clinically significant. This surprising difference may
be due to congenital, or unmeasured comorbidities, suggesting that provider judgment
in ECMO candidate selection may not be effective, as providers may overvalue certain
demographic factors, such as age, in selecting patients to cannulate for ECMO. This
further emphasizes the need for systematic models and evidence-based candidate selection,
rather than relying on individual provider judgment. In today's resource-limited ICUs,
this data is of increasing importance to help providers be aware of the differences
between their expectations, and true clinical prognosis of survival. These findings
are in direct contrast to the SAVE score. This serves to emphasize the role provider
judgment plays in ECMO initiation and emphasizes the necessity for more objective,
quick, and accessible clinical tools in candidate selection. Larger studies should
seek to unpack specific disease processes in younger ECMO candidates to identify which
disease processes are associated with improved or worsened outcomes.
Differences between central and peripheral ECMO are surprising. These differences
may be due to the selection of candidates for cardiac surgery prior to initiation
of ECMO or may be due to differences in artery and vein selection. It is possible
that central ECMO offers reduced rates of complications that have been shown to increase
mortality, as lack of differential in ventilator support, and duration of ECMO support
suggest that this difference in mortality is not secondary to variance in underlying
disease severity, but this is in contrast with prior research that showed increased
rates of limb ischemia in central VA-ECMO.22 This difference may be due to differences in fluid dynamics, leading to improved
coronary perfusion,23 or higher rates of post-transplant ECMO support but the small patient population
in this cohort receiving a heart transplant (n = 19) and durable mechanical support
(n = 7) suggests that a different mechanism underlies this difference in survival.
Additionally, the difference in rates of cardiomyotomy are understandable, due to
the nature of central ECMO, but due to the lack of association with survival, this
difference alone does not explain the difference in survival to hospital discharge
between central and peripheral ECMO support. Further research is necessary to elucidate
the mechanism of this differential.
Although providers may be hesitant to initiate ECMO on obese patients due to difficulty
in cannulation, and high rates of comorbidities,24 prior studies on VV-ECMO have shown no difference in survival to discharge based
on BMI classification.24,25 This study further adds to that body of evidence, evaluating the role of VA-ECMO,
and showed no significance in outcome prediction on BMI. (Table 3) This is important as it rejects the stigma associated with obese patients, allowing
for optimum care. Further research also needs to be done to support this finding within
additional patient cohorts. In this study, sex did not show a significant prediction
in outcome. Such findings are consistent with other ECMO predictor models, ENCOURAGE,
where they found no difference in survival between sexes.26
This study adds to the ability of providers to make evidence-based decisions during
candidate selection for VA-ECMO cannulation. Itsupports the idea that BMI may not
be an independent factor associated with outcome prognosis, while other pertinent
medical history, smoking history, and dialysis history may be important in selecting
patients who will have favorable outcomes after VA-ECMO support.
Summary – Accelerating Translation
Title: Prognostic Factors of Survival in Veno-Arterial ECMO Patients: A Multivariable Logistic
Regression Analysis
Problem to Solve
Extracorporeal membranous oxygenation (ECMO) is a method of providing support to a
patient in heart failure, who's heart has a weakened ability to circulate blood. This
support, however, is invasive, risky, and is associated with a high rate of mortality.
Additionally, due to the complexity of ECMO, it requires higher than normal levels
of staffing, and training. Due to the resource limitations on the medical system,
identifying patients who will benefit from ECMO support, and are most likely to survive
is of critical importance. These limitations are at odds with the increasing need
for ECMO support. As a result of this conflict, novel strategies must be developed
to identify ideal candidates for ECMO support, and elucidate prognostic markers for
favorable patient outcomes.
Aim of Study
This study seeks to use demographic and medical history to identify patients who are
most likely to survive and benefit most from ECMO support. The importance of creating
a model that is based on readily available patient information prior to ECMO initiation
rather than variables that present during the duration of the support is central to
the aims of this research.
Methodology
All patients who received ECMO support between 2016 and 2020 at a single large center
were retrospectively included in this study. A model to isolate the effect of each
variable on patient survival was generated, allowing the researchers to identify the
impact of each variable individually on the outcome.
Results
There was an increase in odds of survival to hospital discharge in patients who were
older. There was a decrease in odds of survival to hospital discharge in patients
who had a prior cardiac arrest. Of patients who survived to hospital discharge, their
average age was 51.4+/-14.0 compared to those who did not survive, with an average
age of 57.3+/- 16.5, a statistically significant difference. Patients who survived
to hospital discharge were less likely to have smoked. Patients who survived to hospital
discharge had lower likelihood of a prior cardiac arrest (13.0% v. 29.2%, respectively;
p = 0.0002). No other associations were noted.
Conclusion
This study shows older age, no history of dialysis, and no history of cardiac arrest
as protective prognostic factors leading to discharge from the hospital. Differences
between central ECMO placed in the chest and peripheral ECMO placed in limbs and neck
are surprising. It is possible that that central ECMO offers reduced rates of complications
that have been shown to increase mortality, as lack of differential in ventilator
support, and duration of ECMO support suggest that this difference in mortality is
not secondary to variance in underlying disease severity, but this is in contrast
with prior research that showed increased rates of limb ischemia in central VA-ECMO.
This study supports existing predictors of survival in patients receiving ECMO, and
importantly notes poorer survival in patients with an age greater than 55, history
of smoking, history of dialysis, and history of cardiac arrest. These factors can
potentially help guide selection of patients for ECMO in the current resource limited
ICU setting.
Acknowledgments
Jay Hwang MD, Igor Gosev MD.
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions
Conceptualization: ASJ, RB, CL. Data Curation: ASJ, GOIV, WW, RB, CL. Formal Analysis:
ASJ, RB, CL. Investigation: ASJ, WW, RB, CL. Methodology: ASJ, RB, CL. Project Administration:
ASJ, RB, CL. Resources: CL. Software: ASJ, CL. Supervision: CL. Validation: ASJ, CL.
Visualization: ASJ, CL. Writing - Original Draft: ASJ, GOIV, RB, CL. Writing - Review
Editing: ASJ, GOIV, WW, RB, CL.
References
1. Chakaramakkil MJ, Sivathasan C. ECMO and Short-term Support for Cardiogenic Shock in Heart Failure. Curr Cardiol Rep. 2018;20(10):87.
2. Paolone S. Extracorporeal Membrane Oxygenation (ECMO) for Lung Injury in Severe Acute Respiratory
Distress Syndrome (ARDS): Review of the Literature. Clin Nurs Res. 2017;26(6):747–62.
3. Telukuntla KS, Estep JD. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist Debakey Cardiovasc J. 2020;16(1):27–35.
4. Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD, et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(6):698–716.
5. Hoyler MM, Kumar S, Thalappillil R, White RS, Tam CW. VV-ECMO usage in ARDS due to COVID-19: Clinical, practical and ethical considerations. J Clin Anesth. 2020;65:109893.
6. Murugappan KR, Walsh DP, Mittel A, Sontag D, Shaefi S. Veno-venous extracorporeal membrane oxygenation allocation in the COVID-19 pandemic. J Crit Care. 2021;61:221–6.
7. Giraud R, Legouis D, Assouline B, De Charriere A, Decosterd D, Brunner ME, et al. Timing of VV-ECMO therapy implementation influences prognosis of COVID-19 patients. Physiol Rep. 2021;9(3):e14715.
8. Hong X, Xiong J, Feng Z, Shi Y. Extracorporeal membrane oxygenation (ECMO): does it have a role in the treatment of
severe COVID-19? Int J Infect Dis. 2020;94:78–80.
9. Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after
veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36(33):2246–56.
10. Chen WC, Huang KY, Yao CW, Wu CF, Liang SJ, Li CH, et al. The modified SAVE score: predicting survival using urgent veno-arterial extracorporeal
membrane oxygenation within 24 hours of arrival at the emergency department. Crit Care. 2016;20(1):336.
11. Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis
of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care. 2017;21(1):45.
12. Bateman RM, Sharpe MD, Jagger JE, Ellis CG, Solé-Violán J, López-Rodríguez M, et al. 36th International Symposium on Intensive Care and Emergency Medicine : Brussels, Belgium. 15-18 March 2016. Crit Care. 2016;20(Suppl 2):94.
13. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory
failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP)
score. Am J Respir Crit Care Med. 2014;189(11):1374–82.
14. Orsini J, Blaak C, Yeh A, Fonseca X, Helm T, Butala A, et al. Triage of Patients Consulted for ICU Admission During Times of ICU-Bed Shortage. J Clin Med Res. 2014;6(6):463–8.
15. Dalia AA, Ortoleva J, Fiedler A, Villavicencio M, Shelton K, Cudemus GD. Extracorporeal Membrane Oxygenation Is a Team Sport: Institutional Survival Benefits
of a Formalized ECMO Team. J Cardiothorac Vasc Anesth. 2019;33(4):902–7.
16. Fitzgerald DC, Darling EM, Cardona MF. Staffing, Equipment, Monitoring Considerations for Extracorporeal Membrane Oxygenation. Crit Care Clin. 2017;33(4):863–81.
17. Bjelic M, Kumar N, Gu Y, Chase K, Paic F, Gosev I. Cause of In-Hospital Death After Weaning from Venoarterial-Extracorporeal Membrane
Oxygenation. J Intensive Care Med. 2022:8850666221086839.
18. Ayers B, Bjelic M, Kumar N, Wood K, Barrus B, Prasad S, et al. Long-term renal function after venoarterial extracorporeal membrane oxygenation. J Card Surg. 2021;36(3):815–20.
19. Amin F, Lombardi J, Alhussein M, Posada JD, Suszko A, Koo M, et al. Predicting Survival After VA-ECMO for Refractory Cardiogenic Shock: Validating the
SAVE Score. CJC Open. 2021;3(1):71–81.
20. Appelt H, Philipp A, Mueller T, Foltan M, Lubnow M, Lunz D, et al. Factors associated with hemolysis during extracorporeal membrane oxygenation (ECMO)-Comparison
of VA-versus VV ECMO. PLoS One. 2020;15(1):e0227793.
21. Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review
of the pathophysiology. Crit Care. 2016;20(1):387.
22. Blakeslee-Carter J, Shao C, LaGrone R, Gonzalez-Sigler I, Sutzko DC, Pearce B, et al. Vascular complications based on mode of extracorporeal membrane oxygenation. J Vasc Surg. 2022;75(6):2037–46.e2.
23. Malinowski D, Fournier Y, Horbach A, Frick M, Magliani M, Kalverkamp S, et al. Computational fluid dynamics analysis of endoluminal aortic perfusion. Perfusion. 2022:2676591221099809.
24. Galvagno SM, Jr., Pelekhaty S, Cornachione CR, Deatrick KB, Mazzeffi MA, Scalea TM, et al. Does Weight Matter? Outcomes in Adult Patients on Venovenous Extracorporeal Membrane
Oxygenation When Stratified by Obesity Class. Anesth Analg. 2020;131(3):754–61.
25. Swol J, Buchwald D, Strauch JT, Schildhauer TA, Ull C. Effect of body mass index on the outcome of surgical patients receiving extracorporeal
devices (VV ECMO, pECLA) for respiratory failure. Int J Artif Organs. 2017:0.
26. Muller G, Flecher E, Lebreton G, Luyt C-E, Trouillet J-L, Bréchot N, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO
for acute myocardial infarction with cardiogenic shock. Intensive Care Medicine. 2016;42(3):370–8.
Andrew Jones, 1 BA, 3rd year medical student. University of Rochester School of Medicine and Dentistry.
Rochester, NY.
George Olverson, 2 BS, 3rd year medical student. University of Rochester School of Medicine and Dentistry.
Rochester, NY.
Wayne Wong, 3 BS, 4th year medical student. University of Rochester School of Medicine and Dentistry.
Rochester, NY.
Rohun Bhagat, 4 MD, Recent Alumni. University of Rochester School of Medicine and Dentistry. Rochester,
NY.
Clauden Louis, 5 MD, Chief Resident, Division of Cardiac Surgery, Department of Surgery. University
of Rochester Medical Center. Rochester, NY.
About the Author: Andrew received his bachelors in Epidemiology from University of Rochester, and is
a current third year medical student at University of Rochester School of Medicine
and Dentistry. He has a strong interest in cardiac care, cardiac surgery, and mechanical
circulatory support, particularly ECMO and LVAD support.
Correspondence: Clauden Louis. Address: 601 Elmwood Ave, Rochester, NY 14642, United States. Email:
claudenlouis@gmail.com
Editor: Francisco J. Bonilla-Escobar;
Student Editors: Johnmark Boachie, Joseph Tonge, Ahmed Nahian & Hang-Long (Ron) Lixx;
Proofreader: Amy Phelan;
Layout Editor: Ana Maria Morales;
Process: Peer-reviewed
Supplementary Material
Further Univariate Testing and Normalcy Testing
Supplementary Table 1
Survival to ECMO Explant: Univariate Analysis
| Characteristic |
All Patients (n=410) |
Survived to ECMO Explant (n = 181) |
Died on ECMO support (n = 229) |
p-Value |
| Age at Hospitalization (Mean; Years Old) +/- std dev |
55.2 +/- 15.9 |
53.3 +/- 14.8 |
56.8 +/- 16.6 |
0.0005* # |
| Female (%) |
31.71% |
28.73% |
34.06% |
0.249 |
| BMI (kg/m2)
|
30.9 +/- 7.3 |
30.7 +/- 6.7 |
31 +/- 7.7 |
0.79 # |
| Atrial Fibrillation (%) |
26.10% |
24.86% |
27.07% |
0.612 |
| Hypertension (%) |
63.41% |
64.64% |
62.45% |
0.647 |
| Diabetes Mellitus (%) |
32.68% |
34.25% |
31.44% |
0.547 |
| COPD (%) |
15.12% |
12.71% |
17.03% |
0.225 |
| Smoking (%) |
39.76% |
43.65% |
17.03% |
0.152 |
| Dialysis (%) |
39.76% |
43.65% |
36.68% |
0.801 |
| Cardiac Arrest (%) |
76.59% |
87.29% |
68.12% |
< 0.0001* |
| Nonischemic Cardiomyopathy (%) |
43.92% |
51.11% |
38.12% |
0.032* |
Legend:
*Indicates statistical significance of p-value.
#indicates continuous variables that were determined to be non-normally distributed
and thus tested with a Mann-Whitney U test instead of a Welch two-sample two-sided
t-test.
Supplementary Table 2.
Survival to ICU Discharge: Univariate Analysis.
| Characteristic |
All Patients (n=410) |
Survived to ICU Discharge (n = 152) |
Died in ICU (n = 258) |
p-Value |
| Age at Hospitalization (Mean; Years Old) +/- std dev [min, max] |
55.2 +/- 15.9 |
51.6 +/- 14.2 |
57.4 +/- 16.5 |
< 0.0001* # |
| Female (%) |
31.71% |
29.61% |
32.95% |
0.483 |
| BMI (kg/m2)
|
30.9 +/- 7.3 |
30.7 +/- 6.8 |
31 +/- 7.5 |
0.832 # |
| Atrial Fibrillation (%) |
26.10% |
25.66% |
26.36% |
0.876 |
| Hypertension (%) |
63.41% |
62.50% |
63.95% |
0.768 |
| Diabetes Mellitus (%) |
32.68% |
33.55% |
32.17% |
0.773 |
| COPD (%) |
15.12% |
11.84% |
17.05% |
0.155 |
| Smoking (%) |
39.76% |
48.03% |
17.05% |
0.009* |
| Dialysis (%) |
39.76% |
48.03% |
34.88% |
0.207 |
| Cardiac Arrest (%) |
76.59% |
86.84% |
70.54% |
d< 0.0001* |
| Nonischemic Cardiomyopathy (%) |
43.92% |
54.67% |
37.55% |
0.004* |
Legend:
*Indicates statistical significance of p-value.
#indicates continuous variables that were determined to be non-normally distributed
and thus tested with a Mann-Whitney U test instead of a Welch two-sample two-sided
t-test.
Normalcy Testing for Univariate Continuous Variables:
Age:
Shapiro Wilkes: W = 0.96458, P < 0.0001
BMI:
Shapiro Wilkes: W = 0.94943, P < 0.0001
Cite as
Jones A, Olverson IV G, Wong W, Bhagat R, Louis C. Prognostic Factors of Survival
in Veno-Arterial ECMO Patients: A Multivariable Logistic Regression Analysis. Int
J Med Stud. 2023 Oct-Dec;11(4):285-93.
Copyright © 2023 Andrew Jones, George Olverson IV, Wayne Wong, Rohun Bhagat, Clauden
Louis
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 11, NUMBER 4, November 2023
