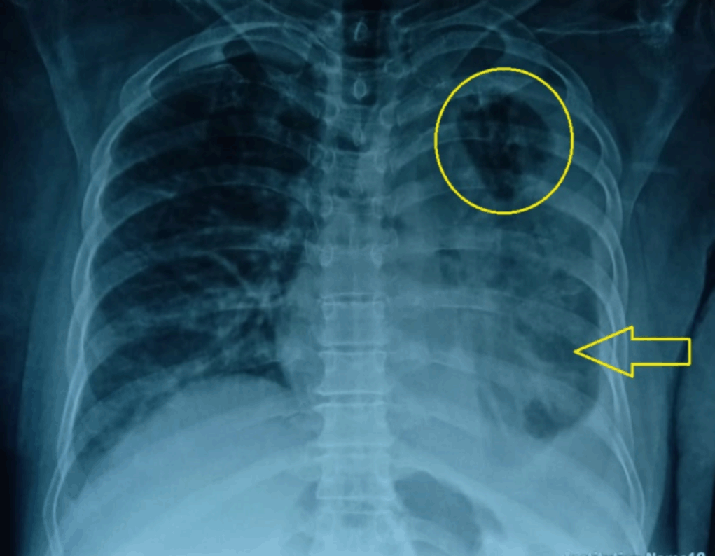
Chest X-ray Showing Cavitary Lesion (shown by a Circle) and Consolidation in the Left Lung (shown by an Arrow).
Highlights:Muqsit Ali Shaukat1, Muhammad Fahad Ali2, Ahmed Irtaza3, Shehroz Yar Khan4, Shad Muhammad Khan5, Sohail Babar6
doi: http://dx.doi.org/10.5195/ijms.2024.1713
Volume 12, Number 2: 212-218
Received 25 09 2022; Rev-request 25 01 2023; Rev-request 21 05 2023; Rev-recd 20 02 2023; Rev-recd 04 01 2024; Accepted 20 06 2024
ABSTRACT
Background:Tuberculosis (TB) presents with productive cough, hemoptysis, chest pain, fever, weight loss, and night sweats. Anti-tuberculosis treatment (ATT) can affect various organs, including the liver and kidneys. ATT-induced acute kidney injury (AKI) presents with fever, rash, nausea, vomiting, diarrhea, and abdominal pain. It occurs due to type 2 or 3 hypersensitivity and affects individuals who have previously used rifampicin or are currently using it intermittently.
Case:An 60-year-old lady was diagnosed with TB and started on ATT. After a few days, she complained of reduced food intake and vomiting, yellow discoloration of the skin, abdominal pain and distention, and limb swelling. She was diagnosed as a case of ATT-induced AKI. She didn't have past exposure to rifampicin and was continuously using it this time.
Conclusion:The key learning point from this case is that ATT-induced AKI can develop even when used in a continuous dosing regime and upon first time exposure despite no history of past exposure. This prompts vigilance in monitoring renal function in patients being started on ATT regimen. This is becasuse, ATT-induced AKI poses risk to patient's life and there is a possibility of developing resistance to anti-tuberculous therapy as a result of discontinuation of treatment. Furthermore, our case suggests that, in addition to immune-mediated mechanisms described in literature for ATT-induced AKI, other pathophysiological mechanisms might also be linked to this pathology and need further research for better understanding and optimization of treatment strategies.
Tuberculosis (TB) has ravaged humankind and has remained endemic and epidemic all over the globe.1 In 2021, about 10.6 million people, including those living with acquired immune deficiency syndrome (AIDS), were inflicted by TB, and 1.6 million died.2 With the advent of effective anti-tuberculosis therapy (ATT) including rifampicin, isoniazid, pyrazinamide, and ethambutol, the future for patients with tuberculosis seemed promising. However, this was cut short as ATT started showing side effects, and the morbidity related to these side effects was significant. Among these side effects, hepatitis, acute kidney injury (AKI), and optic injury are note-worthy, while rash, pyrexia, and gastrointestinal upset are also seen.3 Rifampicin is a vital player in the treatment of TB. The main side effect of rifampicin is hepatotoxicity, while acute kidney injury is less commonly noted. The pathophysiology that seems to be at play behind this side effect is a type 2 and type 3 hypersensitivity reaction mediated by anti-rifampicin antibodies.4,5
Although AKI is a rare complication of ATT, it does delay the treatment of TB and can thus lead to the development of resistance.
The same can be said for liver dysfunction caused by these drugs, as it inevitably leads to a halt or change in the treatment regimen.4 The most common manifestations of renal dysfunction are skin rash, gastrointestinal upset, fever, and hematuria. The common laboratory findings include raised eosinophil count, anemia, and elevated creatinine.3 Patients inflicted by hepatitis due to ATT complain of general malaise, anorexia, nausea, vomiting, fever, skin rash, and pruritus, while the laboratory results show raised transaminases and serum bilirubin.6 Despite this, most patients recover following stoppage or change of the treatment regime.3
Our study presents a unique case of ATT-induced AKI, who presented with chief complaints of reduced food intake and vomiting, jaundice, abdominal pain and distention, and limb swelling. Our case proposes that some other pathophysiological mechanisms might also be linked to causing acute kidney injury in setting of exposure to rifampicin as our patient developed AKI with first time exposure to rifampicin and secondary to continuous usage of rifampicin instead of using it intermittently. Liver function tests were also deranged, suggesting the presence of hepatitis due to ATT. She also developed hypertensive urgency and hypoglycemia as well, which were not previously reported.
A 60-year-old female patient with the past medical history of hypertension, uncontrolled with oral anti-hypertensives, and diabetes mellitus, controlled with oral hypoglycemics, presented with reduced food intake and vomiting, yellow discoloration of the skin, diffuse abdominal pain and bilateral upper and lower limb swelling for four days. The patient had a sudden onset of progressive yellow discoloration of the skin. Her urine was yellowish, and she had no pale stools. She had a gradual onset of abdominal pain with no radiation and no aggravating and relieving factors. The patient also complained of abdominal distention and limb swelling. There was no history of facial puffiness, dyspnea, orthopnea, and paroxysmal nocturnal dyspnea.
For the last two months, she had intermittent, high-grade fever associated with rigors and chills, productive cough with no hemoptysis, and chest pain. She was started on ATT consisting of rifampicin, isoniazid, pyrazinamide, and ethambutol 15 days ago by a local physician as her chest x-ray showed a cavitary lesion and consolidation in the left lung (Figure 1) and the sputum acid-fast bacillus stain was positive.
Figure 1.Chest X-ray Showing Cavitary Lesion (shown by a Circle) and Consolidation in the Left Lung (shown by an Arrow).

At the time of admission into the medical unit, her pulse was 92 beats per minute, her blood pressure was 150/90 mmHg, temperature was 98 degrees Fahrenheit, respiratory rate was 12 per minute, and random blood sugar was 119 mg/dl. Her weight was recorded to be 95 kilogram. General physical examination revealed yellow sclera, pale conjunctivae, and pitting edema of lower limbs up to mid-shins. Pitting edema of bilateral upper limbs was also seen. Systemic examination revealed abdominal distention with shifting dullness.
The rest of the physical examination was unremarkable. After case discussion with senior registrar on-duty, patient was catheterized to measure her urine output and baseline investigations were ordered. Subsequent record of her urine output came to be 380 ml in first 12 hours of admission i.e. she was oliguric (<0.5 ml/kg/hr). Her laboratory investigations are given in Table 1.
Table 1.Laboratory Investigations of the Patient at Admission.
| Name of Exam | Result | Normal range | Unit |
|---|---|---|---|
| Sodium | 122 (↓) | 135–150 | mmol/L |
| Potassium | 4.64 | 3.5–5.1 | mmol/L |
| Chloride | 81 (↓) | 96–112 | mmol/L |
| Blood Urea | 186 (↑) | 18–45 | mg/dl |
| Creatinine | 8.8 (↑) | 0.42–1.06 | mg/dl |
| Total Bilirubin | 1.3 (↑) | 0.1–1.0 | mg/dl |
| Alanine Transaminase | 208 (↑) | 10–50 | IU/L |
| Alkaline Phosphatase | 222 (↑) | 35–104 | IU/L |
| Serum Albumin | 2 (↓) | 3.4–5.4 | g/dl |
| Prothrombin time; | 21.3 (↑) | 12 | seconds |
| Activated partial thromboplastin time | 39.8 (↑) | 28 | seconds |
| White Blood Cells | 20.35 (↑) | 4-nov | ×103/ul |
| Red blood Cells | 4.86 | 4-jun | ×103/ul |
| Hemoglobin | 11.2 (↓) | 11.5–17.5 | g/dl |
| Hematocrit | 32.2 (↓) | 36–54 | % |
| Mean Corpuscular Volume | 66.3 (↓) | 76–96 | fL |
| Mean Corpuscular Hemoglobin (MCH) | 23 (↓) | 27–33 | pg |
| MCH Concentration | 34.8 | 33–35 | g/dl |
| %Red Blood Cell Distribution width | 14.6 (‑) | 11.5–14.5 | % |
| Platelets | 628 (↑) | 150–450 | ×103/ul |
| %Neutrophils | 89.6 (↑) | 40–75 | % |
| %Lymphocytes | 6 (↓) | 20–45 | % |
| #Neutrophils | 18.23 (↑) | 1.9–8 | ×103/ul |
| C Reactive Protein | 10.708 (↑) | <0.5 | mg/dl |
| Virology | |||
| Hepatitis B surface antigen (By ICT) | Negative | ||
| Anti-Hepatitis C Virus (By ICT) | Negative | ||
| Anti-Human immunodeficiency Virus (By ICT) | Negative | ||
| Blood Gas Analysis | |||
| pH (arterial blood) | 7.216 (↓) | 7.35–7.45 | |
| pCO2 | 51.0 (↑) | 35–45 | mmHg |
| pO2 | 48 (↓) | 75–100 | mmHg |
| HCO3 | 20.7 (↓) | 24–27 | mmol/L |
| Lactate Dehydrogenase | 777 (↑) | 80–235 | IU/L |
| Creatine Kinase-MB | 28 (↑) | <25 | IU/L |
| Troponin I | 0.1 | <0.6 | ng/ml |
| Hemoglobin A1c | 14.5 (↑) | 4.6–6.56 | % |
| Urine | |||
| pH | 6 | 4.5–8.0 | |
| Protein | ++ (↑) | Negative | |
| Urobilinogen | Nil | Normal | |
| Pus/White Blood Cells | 6–8 (↑) | 0–5/Hpf | |
| Red Blood Cells | Numerous (↑) | 0–5/Hpf | |
| Epithelial Cells | Few | 0–10/Hpf | |
| Yeast cells | + (↑) | Negative | |
| Pleural Fluid | |||
| Volume | 2 milliliters | ||
| Turbidity | Slight | ||
| Clot | Nil | ||
| Color | Straw | ||
| Protein | 1.6 | ||
| Cell Count | 15/mm3 | ||
| Red Blood Cells Count | 3200/mm3 | ||
| Neutrophils | 10% | ||
| Lymphocytes | 90% | ||
| Gram Stain | No Micro-Organisms seen | ||
| Ziehl Neelsen Stain | No AFB seen | ||
Ultrasound of the abdomen and pelvis showed coarse parenchymal echotexture with serrated margins of liver, increased echogenicity in both kidneys, moderate abdominopelvic ascites, and bilateral mild to moderate pleural effusion. A Pleural tap was done, whose findings are given in Table 1. During her stay at the hospital, her blood pressure was once recorded to be 190/120 mmHg, and random blood glucose level was recorded to be 65 mg/dl with both being managed appropriately. We followed liver function tests and renal function tests over several days. Values of blood urea nitrogen, serum creatinine, total bilirubin, alanine transaminase, and alkaline phosphatase are given in Table 2.
Table 2.Showing Values of Blood Urea Nitrogen, Serum Creatinine, Total Bilirubin, Alanine Transaminase, and Alkaline Phosphatase over the Course of Several Days Starting from the Day of Admission.
| Days | Blood Urea Nitrogen (milligrams per deciliter) | Creatinine (milligrams per deciliter) | Total Bilirubin (milligrams per deciliter) | Alanine Transaminase (milligrams per deciliter) | Alkaline Phosphatase (milligrams per deciliter) |
|---|---|---|---|---|---|
| 1 | 186 | 8.8 | 1.3 | 208 | 222 |
| 8 | 78 | 7.6 | 0.5 | 17 | 217 |
| 15 | 53 | 5.6 | 1.6 | 12 | 159 |
| 22 | 50 | 4.5 | 1.5 | 7 | 154 |
| 29 | 80 | 5.9 | 1.1 | 15 | 153 |
| 36 | 92 | 6.3 | 1.8 | 5 | 256 |
| 37 | 74 | 4.9 | n/a | n/a | n/a |
| 38 | 64 | 4.8 | n/a | n/a | n/a |
| 39 | 80 | 4.8 | n/a | n/a | n/a |
| 41 | 71 | 4.2 | 0.7 | 12 | 218 |
| 42 | 75 | 3.8 | 0.8 | 10 | 227 |
| 43 | 64 | 3.5 | n/a | n/a | n/a |
Our patient lacked a record of baseline serum creatinine value. She had a urine output of less than 0.5 ml/kg/hr for more than 12 hours and a serum creatinine value of 8.8 mg/dl. Based upon this data and the need for hemodialysis in this patient as described later on in this text, she was classified into “Injury” class in RIFLE criteria and Stage 3 of AKIN criteria. Table 3 and Table 4. After case discussion with ward seniors and consultant, she was then put on modified ATT regimen (Isoniazid, Ethambutol, and Pyrazinamide) starting from 3rd day of her admission.
Table 3.Rifle Criteria for Acute Kidney Injury24
| Stage | GFR Criteria | UO Criteria |
|---|---|---|
| Risk | SCr increased 1.5–2 times baseline or GFR decreased >25% | UO < 0.5 mL/kg/h < 6 h |
| Injury | SCr increased 2–3 times baseline Or GFR decreased >50% | UO < 0.5 mL/kg/h >12 h |
| Failure | SCr increased >3 times baseline or GFR decreased 75% or SCr ≥4 mg/dL; acute rise ≥0.5 mg/dL | UO < 0.3 mL/kg/h 24 h (oliguria) or anuria 12 h |
| Loss of Function | Persistent acute renal failure: complete loss of kidney function >4 weeks (requiring dialysis) | |
| ESRD | Complete loss of kidney function >3 months (requiring dialysis) | |
The AKIN Staging System of Acute Kidney Injury.24
| Stage | Serum Creatinine (SCr) | Urine Output (UO) |
|---|---|---|
| 1 | ↑ SCr ≥26.5 μmol/L (≥0.3 mg/dL) or ↑SCr ≥150 a 200% (1.5 a 2×) | <0.5 mL/kg/h (>6 h) |
| 2 | ↑ SCr >200 a 300% (>2 a 3×) | <0.5 mL/kg/h (>12 h) |
| 3b | ↑ SCr >300% (>3×) or if baseline SCr ≥353.6 μmol/L (≥4 mg/dL) ↑SCr ≥44.2 μmol/L (≥0.5 mg/dL) | <0.3 mL/kg/h (24 h) or anuria (12 h) |
Legend:
a Stage 3 also includes patients requiring RRT independent of the stage (defined by SCr and/or UO) they are in at the moment they initiate RRT.Hemodialysis sessions were included in her management plan due to fluid-overload state of the patient and she had her first session on the 4th day of admission followed by six more sessions (one per week) until her renal profile plateaued at serum creatinine value of 3.5 to 4.2 mg/dl (refer to Table 2; serum creatinine values from day 1 to 43 of admission). Remaining inpatient treatment given to the patient is summarized in Table 5.
Table 5.Treatment Given to the Patient in the Hospital.
| Drug | Route | Dose | Frequency |
|---|---|---|---|
| Alprazolam (ALP) | Oral | 0.25 mg | Once a day |
| Amlodipine (Lodopin) | Oral | 5 mg | Once a day |
| Cefoperazone (Sulzon) | Intra Venous | 2 g | Twice a day |
| Dialysis | 6 sessions were done during her stay in the hospital | ||
| Ethambutol | Oral | 400 mg | Once a day |
| Hypertonic Dextrose 25% | Intra Venous | 2 ampules | |
| Insulin Glargine (Lantus) | Subcutaneous | 10 unit | Once a day |
| Insulin Regular (Humulin R) | Subcutaneous | 8 unit | Three times a day |
| Metoclopramide (Maxolon) | Intra Venous | 10 mg | Three times a day |
| Moxifloxacin (Moxiget) | Oral | 400 mg | Once a day |
| Omeprazole (Risek) | Intra Venous | 40 mg | Once a day |
| Isoniazid | Oral | 300 mg | Once a day |
| Pyrazinamide | Oral | 1200 mg | Once a day |
| Salt Free Albumin | Intra Venous | 100 ml | Twice a day |
Given the raised white blood cell count of our patient on arrival (20.35 ×103/ul) with 89.6 % neutrophils (neutrophilic leukocytosis), the patient was commenced on broad spectrum anti-microbial coverage using cefoperazone/sulbactam to provide gram positive and gram negative coverage and moxifloxacin to cover the respiratory microbes including atypical bacteria. The decision to use these specific agents was based on senior consultation keeping in view the availability of agents in the hospital pharmacy.
After recording adequate urine output (> 1 ml/kg/hr) and patient becoming clinically and vitally stable, she was discharged with the advice to continue her modified regimen of ATT and follow up in medicine and nephrology outpatient departments follow-up clinical evaluation, urinalysis, and assessment of renal profile and serum electrolytes and optimization of management of her comorbidities in accordance with post-discharge care for AKI patients as proposed by Tsang JY.7 She was also registered with regional TB center for appropriate and adequate management of her condition.
In this article, we discuss a case of active pulmonary TB in a 60-year-old woman who was tested positive for sputum acid-fast bacillus. She was treated with first line ATT and after 11 days, experienced a reduced food intake and vomiting, progressive yellow discoloration of the skin, abdominal pain and distention and swelling in her limbs for four days. Eventually, she was diagnosed with AKI using the AKIN/RIFLE criteria. Due to acute onset of AKI following initiation of ATT and the absence of any other predisposing factor, it was clinically diagnosed as a case of ATT-induced AKI.
TB, a contagious disease, is caused by a bacteria known as Mycobacterium tuberculosis. Pulmonary TB usually presents with cough, hemoptysis, chest pain, fever, weight loss, and night sweats.1 First-line ATT consisting of rifampicin, isoniazid (INH), pyrazinamide, and ethambutol is usually the mainstay of treatment.2 While hepatitis, dyspepsia, joint pain, rash, and vision problems are some common adverse effects of ATT,3 AKI is a rare adverse effect.8 Studies have reported this to be caused mostly by rifampicin.5,9 In a retrospective case series from 2006–2016, Sakashita K et al,3 Found that 15 out of 1430 patients with active pulmonary TB on ATT developed AKI; 14 of which were rifampin-induced and one INH-induced. Chogtu B et al,4 described a case of ATT associated with AKI whose serological studies revealed the presence of anti-rifampicin antibodies.4
Various studies have revealed that patients on ATT develop AKI when they have either an intermittent dosing regimen of rifampicin or a history of exposure to rifampicin.4,8–10 Muthukumar T et al.,11 studied twenty five patients of ATT-induced AKI admitted from July 1990 to June 2000. The most common pattern of rifampicin administration that resulted in acute renal failure was intermittent dosing regimen while anemia and thrombocytopenia was observed in 60% of cases.11 The proposed pathophysiology is a type 2 or type 3 hypersensitivity reaction mediated by anti-rifampicin antibodies produced upon first-time exposure to rifampicin. Subsequent exposure after a drug-free interval leads to drug-antibody complexes formation, leading to cellular damage causing renal glomerular and tubular injury.4,5 Furthermore, studies have frequently reported the presence of anemia and thrombocytopenia in ATT-induced AKI cases.5,10 De Vriese AS et al,12 proposed that rifampicin-dependent immunoglobulin G and immunoglobulin M exhibit I antigen specificity, expressed on the surface of red blood cells and renal tubular cells, thus explaining hemolytic anemias and renal injury.12 Emma L Smith et al.13 demonstrated rifampin-dependent antiplatelet antibodies leading to thrombocytopenia.
Our patient continuously used rifampicin without any past exposure to rifampicin. Yet, she developed AKI on the 11th day of ATT without a laboratory picture of hemolytic anemia or thrombocytopenia, thus proposing that another pathophysiological mechanism might also be linked with ATT-induced AKI.8 This presentation of our case is very similar to the published case report of Ata F et al. where a 42-year-old Moroccan lady developed AKI secondary to continuous and uninterrupted rifampicin therapy.14
The majority of cases of ATT-induced AKI present with fever, rash, and gastrointestinal symptoms (nausea, vomiting, diarrhea, and abdominal pain) and flu-like syndrome.5,9 In contrast, our patient experienced only vomiting and abdominal pain. Also, the patient had reduced food intake, yellow discoloration of the skin, abdominal pain with distention, and limb swelling, which can be because of ATT-induced AKI with liver involvement or a different clinical picture of the ATT-induced AKI as described in the literature.15 This shows that clinical presentation of ATT-induced AKI may vary from person to person, warranting regular monitoring of renal function tests before and after starting ATT to detect a complication at an earlier stage. Hematuria and proteinuria were documented in our case in the urinalysis report despite our patient presenting an atypical picture of ATT-induced AKI. These findings are also well-documented in literature,3,5,10 thus suggesting that they can be used as reliable parameters for detecting this etiology of AKI even in patients with the atypical presentation but a clinical history of rifampicin exposure.
Literature review of ATT-induced AKI revealed rifampicin to be the likely cause in most cases.16 Based on this review and after case discussion with ward seniors and consultants, she was started on modified ATT regimen that included isoniazid, ethambutol and pyrazinamide. Considering the signs and symptoms of fluid overloaded state (abdominal distention, bilateral upper and lower limb edema), consensus in round discussion was to start renal replacement therapy in form of hemodialysis.
Our patient was managed by temporarily stopping ATT regimen and arranging hemodialysis sessions until adequate urine output (> 1 ml/kg/hr) and plateaued serum creatinine values were obtained after six weeks. This is in contrast with the case of Ata F et al.14 and their literature review where kidney functions were observed to normalize within three weeks after discontinuation of culprit agents. However, Ata F et al. also mention the use of steroids along with hemodialysis sessions that was not utilized in our case thereby suggesting potential benefit of steroid therapy in speeding recovery in such cases. However, it is important to note that use of steroids for ATT-induced AKI remains a topic of controversy.14
During her stay, our patient developed hypertensive urgency (blood pressure was recorded to be 190/120 without the progression of her abnormalities), for which she was managed with anti-hypertensives. This can be because either the potency of antihypertensive medications is decreased in patients who are on ATT or ATT-AKI induced,17 warranting regular monitoring of blood pressure. Even though our patient was diabetic, she developed an episode of hypoglycemia managed with intravenous 25% dextrose. This can be due to hypoglycemic agents or decreased caloric intake as well.18 Apart from that, hypoglycemia has been reported due to kidney injury.19 Therefore, regular monitoring of blood glucose levels should be done.
Key limitations of this case report that warrant mention are:
From the knowledge we have gathered from our literature review of this case, our hypothetical expectations and the way forward are:
In developing countries like Pakistan, where it becomes difficult to accurately diagnose and treat a complication once it develops and keep appropriate follow-up, the resources can be utilized better if they are focused on preventing a complication. Given the high prevalence of tuberculosis in our region, the use of ATT is widespread, and thus, preventing the development of ATT associated with AKI will be far more effective than treating it once it develops. However, the literature needs a discussion on how to prevent the development of this complication. Further work and study into this topic are required to create detailed guidelines.
Acute kidney injury is a rare side effect of anti-tuberculosis therapy, usually caused by either intermittent use of rifampicin or a history of previous exposure to rifampicin. The pathophysiological mechanism responsible for this is reported to be a type 2 or type 3 hypersensitivity reaction resulting from anti-rifampicin antibodies. Acute kidney injury resulting from anti-tuberculosis therapy usually presents with fever, rash, gastrointestinal symptoms (nausea, vomiting, diarrhea, and abdominal pain), and flu-like symptoms. We present a unique case of anti-tuberculosis therapy-induced acute kidney injury that had a concomitant anti-tuberculosis-induced liver injury as well and presented with the clinical features of reduced food intake and vomiting, yellow discoloration of the skin, abdominal pain and distention, and limb swelling suggesting that clinical suspension of this side effect should be high as signs and symptoms might vary. Rifampicin was used continuously, and this patient had no reported history of rifampicin use, which suggests that another pathophysiological mechanism might be responsible for anti-tuberculosis therapy-induced acute kidney injury instead of type 2 or type 3 hypersensitivity. She developed hypertensive urgency and hypoglycemia during her stay in the hospital, suggesting that vital monitoring should be done in these patients to prevent life-threatening emergency.
None
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: M.A.S., M.F.A., A.I.. Project Administration: M.A.S.. Writing - Original Draft: M.A.S., M.F.A., A.I., S.Y.K., S.M.K., S.B.. Writing - Review Editing: M.A.S., M.F.A., A.I., S.Y.K., S.M.K., S.B..
1. Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191(3):834–44.
2. World Health Organization. Tuberculosis (TB). Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Last updated Nov 7, 2023; cited Feb 07, 2023.
3. Sakashita K, Murata K, Takahashi Y, Yamamoto M, Oohashi K, Sato Y, et al. A case series of acute kidney injury during anti-tuberculosis treatment. Intern Med. 2019;58(4):521–7.
4. Chogtu B, Surendra VU, Magazine R, Acharya PR, Yerrapragada DB. Rifampicin-induced concomitant renal injury and hepatitis. J Clin Dian Res. 2016;10(9):0D18–9.
5. Covic A, Goldsmith DJA, Segall L, Stoicescu C, Lungu S, Volovat C, et al. Rifampicin-induced acute renal failure: A series of 60 patients. Nephrol Dial Transplant. 1998;13(4):924–9.
6. Shang P, Xia Y, Liu F, Wang X, Yuan Y, Hu D, et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China. PLoS One. 2011;6(7):e21836.
7. Tsang JY, Murray J, Kingdon E, Tomson C, Hallas K, Campbell S, et al. Guidance for post-discharge care following acute kidney injury: an appropriateness ratings evaluation. BJGP Open. 2020;4(3):bjgpopen20X101054.
8. Beebe A, Seaworth B, Patil N. Rifampicin-induced nephrotoxicity in a tuberculosis patient. J Clin Tuberc Other Mycobact Dis. 2015;1:13–5.
9. Schubert C, Bates WD, Moosa MR. Acute tubulointerstitial nephritis related to antituberculous drug therapy. Clin Nephrol. 2010;73(6):413–9.
10. Chiba S, Tsuchiya K, Sakashita H, Ito E, Inase N. Rifampicin-induced acute kidney injury during the initial treatment for pulmonary tuberculosis: A case report and literature review. Intern Med. 2013;52(21):2457–60.
11. Muthukumar T, Jayakumar M, Fernando EM, Muthusethupathi MA. Acute renal failure due to rifampicin: a study of 25 patients. Am J Kidney Dis. 2002;40(4):690–6.
12. De Vriese AS, Robbrecht DL, Vanholder RC, Vogelaers DP, Lameire NH. Rifampicin-associated acute renal failure: Pathophysiologic, immunologic, and clinical features. Am J Kidney Dis. 1998;31(1):108–15.
13. Smith EL, Bywater L, Pellicano R, Jenkin GA, Korman TM. Acute Tubular Necrosis and Thrombocytopenia Associated with Rifampin Use: Case Report and Review. Open Forum Infect Dis. 2022;9(7).
14. Ata F, Magboul HMB, Toba HAA, Alfar H, Al Bozom A, Murshed K, et al. Rifampin-induced acute kidney injury and hemolysis: A case report and literature review of a rare condition. Clin Case Rep. 2022;10(12):e6780.
15. Penn Medicine. Acute Kidney Injury (AKI) - Symptoms and Causes. Available from: https://www.pennmedicine.org/for-patients-andvisitors/patient-information/conditions-treated-a-to-z/acute-kidneyinjury. Last updated Nov 4, 2022; cited Sep 14, 2022.
16. Chang CH, Chen YF, Wu VC, Shu CC, Lee CH, Wang JY, et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis. 2014;14(1):23.
17. Sharma AP, Sural S, Gupta A, Garg AX, Gulati S, Sharma RK. Effect of antitubercular medications on blood pressure control in chronic kidney disease patients with tuberculosis: a prospective cohort study. J Nephrol. 2006;19(6):771–7.
18. Arem R. Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am. 1989;18(1):103–21.
19. Fiaccadori E, Sabatino A, Morabito S, Bozzoli L, Donadio C, Maggiore U, et al. Hyper/hypoglycemia and acute kidney injury in critically ill patients. Clin Nutr. 2016(2): 317–21.
20. Beck Jr. LH, Salant DJ. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGraw-Hill Education; 2018.
21. Moussa C, Esbaa S, Rouis H, Sellami N, Hajji M, Houcine Y, et al. Rifampicin-induced acute tubulointerstitial nephritis during pulmonary tuberculosis treatment: A case report. Respirol Case Rep. 2023;11(8):e01190.
22. Moledina DG, Parikh CR. Differentiating acute interstitial nephritis from acute tubular injury: a challenge for clinicians. Nephron. 2019;143(3):211–6.
23. Clarkson MR, Giblin L, O’Connell FP, O’Kelly P, Walshe JJ, Conlon P, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19(11):2778–83.
24. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6(1):8–14.
Muqsit Ali Shaukat, 1 Bachelor of Medicine and Bachelor of Surgery (MBBS). Resident Physician in General Medicine at Federal Government Polyclinic Hospital, Islamabad Pakistan.
Muhammad Fahad Ali, 2 Bachelor of Medicine and Bachelor of Surgery (MBBS). Resident Physician in General Surgery at Pakistan Institute of Medical Sciences (PIMS), Islamabad Pakistan.
Ahmed Irtaza, 3 Bachelor of Medicine and Bachelor of Surgery (MBBS). Resident Physician in General Medicine at Khyber Teaching Hospital, Peshawar.
Shehroz Yar Khan, 4 Bachelor of Medicine and Bachelor of Surgery (MBBS). Resident Physician in General Medicine at Lady Reading Hospital, Peshawar, Pakistan.
Shad Muhammad Khan, 5 Bachelor of Medicine and Bachelor of Surgery (MBBS). Resident Physician in General Medicine at Khalifa Gul Nawaz Teaching Hospital, Bannu, Pakistan.
Sohail Babar, 6 Bachelor of Medicine and Bachelor of Surgery (MBBS). Resident Physician in General Medicine at Mufti Mehmood Memorial Teaching Hospital, Dera Ismail Khan, Pakistan.
Correspondence: Muqsit Ali Shaukat. Address: 44 Luqman Hakeem Rd, G-6/2 G 6/2 G-6, Islamabad, Islamabad Capital. Email: muqsitali@hotmail.com
Editor: Francisco J Bonilla-Escobar; Student Editors: Ahmed Nahian Bahadar Srichawla; Proofreader: Amy Phelan; Layout Editor: Julián A Zapata-Ríos; Process: Peer-reviewed
Cite as Shaukat M A, et al. Continuous Rifampicin Therapy Induced Acute Kidney Injury in a Tuberculous Patient: A Case Report. Int J Med Stud. 2024 Apr-Jun;12(2):212-118.
Copyright © 2024 Muqsit Ali Shaukat, Muhammad Fahad Ali, Ahmed Irtaza, Shehroz Yar Khan, Shad Muhammad Khan, Sohail Babar
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 12, NUMBER 2, June 2024