Original Article
Meloxicam Decreases the Formation of Peritoneal Adhesions in an Experimental Surgical
Model in Rats
Luis Alfredo Hernandez Villarroel1, Henry Fernandez1, Luisa Cesin1
doi: http://dx.doi.org/10.5195/ijms.2017.175
Volume 5, Number 1: 6-13
Received 02 01 2017:
Accepted 19 03 2017
ABSTRACT
Background:
Inflammatory adhesions result from an inflammatory response of the peritoneum during
an intra-abdominal inflammatory process secondary to thermal or mechanical injury,
infection, radiation, ischemia, dissection, abrasion or foreign body reaction. Adhesions
produce consequences such as: infertility, intestinal obstruction, and pelvic-abdominal
pain. The objective of this study is to evaluate the effects of Meloxicam, a selective
cyclooxygenase-2 inhibitor, on the formation of postoperative peritoneal adhesions
in an experimental animal model.
Methods:
Twenty female Wistar rats were submitted to laparotomy. Postoperative peritoneal adhesions
were induced by scorching the serous surface of the colon. The animals were randomly
divided into two experimental groups: one group received Meloxicam intramuscularly
for 7 days, and the other served as a control group. They were sacrificed and evaluated
at 15 days.
Results:
In the animals given Meloxicam, it was observed that a decrease in number (p = 0.018),
severity (p = 0.004), extension (p = 0.011), density (p = 0.023), degree of inflammation
(p = 0.002), vascular proliferation (p = 0.004) and fibrosis (p = 0.029) of adhesions,
compared to the control group.
Conclusion:
In conclusion, this study demonstrated that the administration of Meloxicam intramuscularly
significantly decreases the formation of postoperative peritoneal adhesions and, therefore,
may be useful in their prevention. The effects of Meloxicam could not only be due
to its anti-inflammatory action, but also to its effects on the expression of the
Vascular Endothelial Growth Factor.
Keywords:
Cyclooxygenase 2 Inhibitors;
Experimental Animal Models;
General Surgery;
Non-Steroidal Anti-Inflammatory Agents;
Tissue Adhesions.
Introduction
Peritoneal adhesions are pathological junctions of connective tissue formed between
organs and tissues, and frequently between the omentum, intestines and abdominal wall.
The etiology may be congenital or acquired. The acquired adhesions are classified
postoperatively or postinflammatory. Inflammatory adhesions result from an inflammatory
response of the peritoneum during an intra-abdominal inflammatory process, such as
appendicitis and pelvic inflammatory disease. Post-surgical adhesions develop when
a tissue is injured by surgical manipulation. 1, 2
In a prospective study, 93% of patients with a previous laparotomy had peritoneal
adhesions, and the incidence of read-missions directly related to adhesions varies
from 5% to 20%.3,4 Each year, 400,000 adhesiolysis procedures are performed in the United States, with
a cost in the health system of close to $ 2 trillion in hospitalizations and surgeries.5
Adhesions are the result of tissue trauma that may be the consequence of thermal or
mechanical injury, infection, radiation, ischemia, dissection, abrasion, or foreign
body reaction.6 However, the most important and potential consequences resulting from
the formation of peritoneal adhesions are: infertility, intestinal obstruction, and
pelvic-abdominal pain. They can affect fertility by distorting the attached anatomy
and interfere with the transport of the gamete and embryo. 6
The most serious of the complications caused by adhesions is small bowel obstruction.7 Adhesions account for 56% of postoperative intestinal obstructions.8 This surgical
emergency has a mortality rate of 3-10% for simple obstruction, and up to 30% when
the intestine is necrotic or perforated. In one study, it was evidenced that of 2000
patients submitted to laparotomy 1-2% developed obstruction secondary to peritoneal
adhesions in the same year of surgery. The incidence of intestinal obstruction secondary
to adhesion formation is 1-10% in 4-6 years after appendectomy, 6% in 5 years after
cholecystectomy, 9-25% in 2-10 years following intestinal surgery, and 17-25 % in
5-10 years following proctocolectomy.8
However, peritoneal adhesions are not a new problem, surgeons have studied different
barrier/pharmacological agents to prevent the formation of adhesions.9 In this way, different synthetic barrier methods have been used for its prevention.10.11 Among the pharmacological agents studied are: allopurinol, thymoquinone, phospholipids,
spironolacton e, captopril, heparin has been studied.1, 6, 12-14 Also a variety of steroids and anti-inflammatory agents have been studied, including
aspirin, dexamethasone, methylprednisolone, estrogen, progesterone and budesonide.15 Likewise, the use of hemostatic agents, and the effects of vitamin E and amniotic
membrane on the formation of adhesions have been researched.16, 17 However, none of these pharmacological agents and barrier methods have demonstrated
clinically relevant results in reducing chronic pain, decreased infertility, and rate
of reoperation.18
Meloxicam, is a non-steroidal anti-inflammatory categorized as a selective COX-2 inhibitor.
It is commonly used in the treatment of acute and chronic pain and inflammation.19
The objective of this study is to evaluate the anti-inflammatory effects of Meloxicam
in the formation of postoperative peritoneal adhesions in an experimental animal model.
Therefore, in the present study we proposed the use of an experimental model of adhesion
formation by serosal abrasion of the colon, having a control group without drug administration
and a group to which Meloxicam was given intramuscularly. In light of the above considerations
and by its mechanism of action, it is proposed as an alternative hypothesis that Meloxicam
inhibits the formation of postoperative peritoneal adhesions; and as a null hypothesis,
that Meloxicam does not inhibit the formation of adhesions.
Materials and Methods
An experimental study was carried out in an animal model, in which 20 female rats,
Wistar strain, between 250 and 300 g in weight were used. Two experimental rats were
kept per cage, with food and water to free demand. The bed of each cage was changed
twice a week. They were under cycles of 12 hours light and 12 hours darkness and a
temperature in the experimental laboratory of 22ćC ± 2ćC.
All procedures of the research protocol were carried out strictly taking into account
the principles for the care and use of laboratory animals, according to the bioethics
criteria for the experimentation of the Venezuelan Association for the Science of
Laboratory Animals.20
Before the operation, the experimental animals were randomly distributed into two
groups consisting of 10 animals each: a control group and a study group.
In each experimental rat, we proceeded to perform a surgical procedure with the goal
to induce the adhesion formation process. Each subject was previously anesthetized
by administration of 100 mg/kg Ketamine (Keiran®) and 10 mg/kg Xylazine (Rompun®),
both intramuscularly.
The abdominal skin was disinfected with Povidone Iodine solution (BETADINE®), prior
to the procedure. After, a vertical midline incision measuring 3 cm in length was
made, the large intestine of the rat was exposed and the induction of the process
of formation of peritoneal adhesions was carried out by a technique already described,
which consisted of injuring the serosa of the large intestine by vigorous rubbing
with dry gauze (Figure 1A). The rubbing was maintained until the appearance of hemorrhagic points. This procedure
was performed in 5 segments corresponding to the cecum, 1 ascending colon segment,
1 transverse colon segment, 1 descending colon segment and sigmoid segment. Each segment
was 1 cm in length.
Figure 1.
Some of the Procedures Performed During the Adhesion-Forming Surgery and the Immediate
Postoperative Period of the Rat. A. Exposure of the large intestine of the rat B. The skin and subcutaneous cellular tissue were closed with 4-0 nylon. C. Postoperative immediately, the experimental animal is in an incubator (medix®, model
PC-305) adjusted to 32ćC, enabled for its recovery.
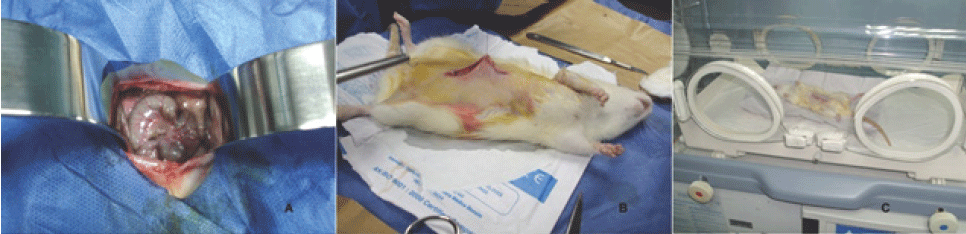
The abdominal wall was closed, suturing the aponeurosis and rectus abdominis muscle
with Polyglactin 910 (Vycril®) 4-0; skin and subcutaneous cellular tissue with 4-0
nylon (Figure 1B). At the end of the surgical procedure, each experimental animal was placed in an
incubator (Medix®, model PC-305) set at 32ćC, where they lasted for 4 hours with the
finality to achieve recovery (Figure 1C).
In terms of treatment, the control group had no therapy applied. The study group,
was administered Meloxicam (Mobic®, Boehringer Ingelheim) intramuscularly, at a dose
of 0.20 mg/kg/day, for 7 days, starting right after the surgery.
All experimental rats were sacrificed at day 15 postoperative, under the effects of
anesthesia already described. 1 mL of 7.5% Potassium Chloride was administered via
intracardiac injection, via a thoracotomy to achieve exposure of the heart.
An abdominal “U” incision was made by lifting the abdominal wall of the experimental
rat with the objective to evaluate the adhesions formed, registering their presence
or absence, formation in unmanipulated organs, and whether there were anterior or
posterior abdominal wall attachments. Likewise, each of the adhesions presented by
the experimental animals of each group was counted.
The degree of severity, dissection and extension were evaluated according to the classification
of Diamond.21 According to the severity, it was considered: Grade 0, without adhesions;
Grade 1, thin and avascular adhesions were evident; Grade 2, vascularized and dense
adhesions were observed; Grade 3, adhesions were firm and cohesive. As for its extension:
Grade 0, without adhesions; Grade 1, less than 25%; Grade 2, between 26% and 50%,
Grade 3, more than 50% of surface. The density was evaluated by the following: Grade
0, without adhesions; Grade 1, the adhesions were released spontaneously upon separation
of the flap; Grade 2, mild to moderate traction was required to separate the adhesions;
Grade 3, those that merited adhesiolysis with scissors. Adhesion tissue samples were
dehydrated, placed in paraffin, and then 3 micron cuts were made with a rotating microtome
(MICROM®). The sections were stained with Haematoxylin and Eosin (H/E) and Masson’s
Trichrome stain. The surgical pieces were analyzed by a blind observer to the procedure,
in the laboratory of Histopathology of the Hospital Complex University Ruíz and Paez,
Ciudad Bolívar, Venezuela.
The histological characteristics of the adhesions were determined according to the
histological classification of Kanbour-Shakir, which evaluates three aspects: fibrosis,
inflammation and vascular proliferation. Firstly, fibrosis is measured by the percentage
of occupation of fibroblasts in a dry field of observation (mild <33%, moderate >
33% and < 66%, severe > 66%, and no fibroblast proliferation). Secondly, inflammation
involves observing the presence of inflammatory cells and classifying it as mild (infiltrating
with occasional giant cells, lymphocytes and plasma cells), moderate (giant cells,
eosinophils and neutrophils) and severe (abundant inflammatory cells and microabscesses)
or absence of an inflammatory component. Lastly, vascular proliferation, defined as
the number of blood vessels present per area in 40x magnification or high powered
field, classified as mild (blood vessels in less than 33% of the field extension),
moderate (in more than 33% and less than 66% of the area) and severe (> 66%) or without
blood vessels.22
Statistical analysis was performed using the SPSS software (version 23; Statistical
Package for the Social Sciences, SPSS Inc, Chicago, IL). The normality of the variables
was evaluated by the Shapiro-Wilk test. For the comparison of the means of each group:
the quantitative variables with a normal distribution were assessed with the Student’s
t-test for independent samples; variables not fulfilling a normal distribution were
evaluated by the Mann-Whitney U-test; and the qualitative variables were evaluated
with the Chi-square test. The confidence interval was set at 95% and the differences
were considered statistically significant when the p-value was ,0.05.
Results
All experimental animals completed the study. No congenital adhesions were evident
in any of the rats in the first surgical intervention. After the surgical procedure,
there were no complications such as wound infections, peritonitis or intestinal obstruction.
Regarding the presence of adhesions formed after the surgical induction procedure,
18 of 20 research animals had at least one adhesion. Peritoneal adhesions were formed
in all rats (10 of 10 animals) belonging to the control group, while in the rats belonging
to the study group (with administration of Meloxicam) the adhesions were evident in
8 of 10 experimental animals.
It was observed that the group of animals that were administered meloxicam after the
surgical procedure two animals had only a single adhesion. The total adhesions per
experimental group were: 32 in the group treated with meloxicam and 183 in the control
group. The mean number of adhesions per group was 18.3 for the control group and 3.2
for the study group (Table 1). These differences were statistically significant (control vs. study group, p =
0.018).
Table 1.
Clinical outcomes when comparing the interventions.
| Characteristic |
Control (n=10) |
Meloxicam (n=10) |
P-value |
| Peritoneal adhesions, mean (SD) |
18.30 (16.45) |
3.20 (2.15) |
0.018 |
| Peritoneal adhesions formed in non-manipulated organs* |
|
|
0.003 |
| Yes, n (%) |
7 (100) |
0 (0) |
0.003 |
| No, n (%) |
3 (23.08) |
10 (76.92) |
0.003 |
| Peritoneal adhesions formed attached to anterior abdominal wall* |
|
|
0.087 |
| Yes, n (%) |
4 (100) |
0 (0) |
0.087 |
| No, n (%) |
6 (37.50) |
10 (62.50) |
0.087 |
| Peritoneal adhesions formed attached to posterior abdominal wall* |
|
|
0.087 |
| Yes, n (%) |
4 (100) |
0 (0) |
0.087 |
| No, n (%) |
6 (37.50) |
10 (62.50) |
0.087 |
7 of 10 animals in the control group presented with adhesions in unmanipulated organs
during the surgical procedure, whereas in the study group none of the experimental
animals presented with adhesions in unmanipulated organs. There was a statistically
significant difference between the two groups, p = 0.001 (Table 2). The presence of
adhesions with anterior or posterior abdominal wall attachment occurred in 4 of 10
animals belonging to the control group. However, this characteristic was not evidenced
in the group of animals who were administered meloxicam. There was a statistical difference
denoting p = 0.025, in relation to adhesions to the anterior and posterior abdominal
wall (Tables 3 & 4).
Regarding the assessment of adhesions, statistically significant differences were
evidenced, according to their severity, extension and density (ease for dissection),
with corresponding p values: p = 0.004, p = 0.011 and p = 0.023, respectively (Figures 2 & 3).
Figure 2.
Degree of severity, density and extent of peritoneal adhesions. *Statistical significance
compared with control group.
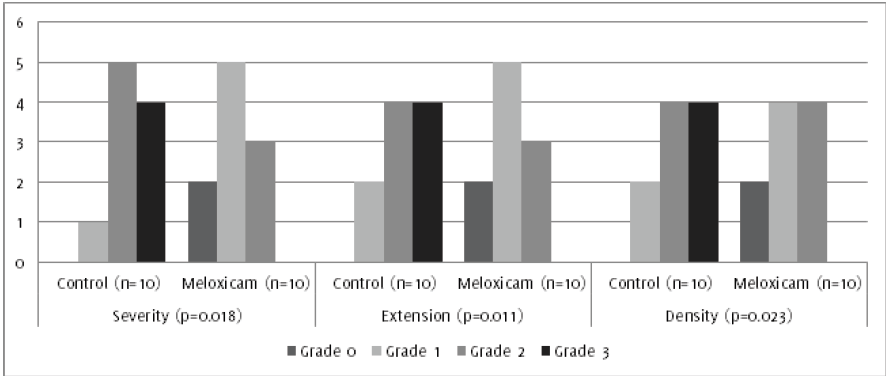
Figure 3.
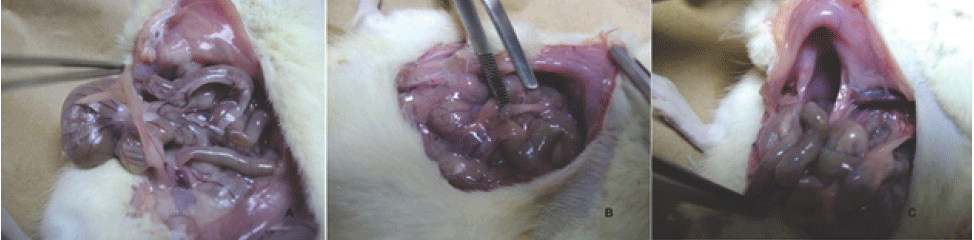
Degrees of Severity of Peritoneal Adhesions Evidenced During Macroscopic Assessment.
A. Rat of the study group (Meloxicam), which demonstrates a loose adhesion that could
be released exclusively with traction. B. Strong and cohesive peritoneal adhesions between several thin intestinal loops, with
a greater than 50% extension of the abdominal cavity, which required adhesiolysis
with scissors for their release. C. Adhesions of the small intestine to the anterior abdominal wall (in the control group
rat) involving more than 50% of the abdominal cavity with a high degree of severity
and impossibility of release, only by adhesiolysis.
In the histopathological study (Figures 4 & 5), the development of different degrees of fibrosis was evidenced, depending on the
treatment group. Animals treated with Meloxicam had a lower degree of fibrosis, and
this difference was statistically significant when compared to the control group (p
= 0.029).
Figure 4.
Degrees of fibrosis, inflammation and vascular proliferation in postoperative peritoneal
adhesions. *Statistical significance compared with control group.
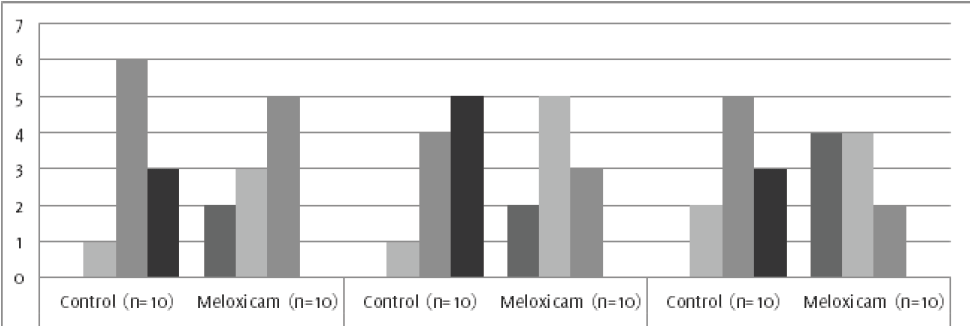
Figure 5.
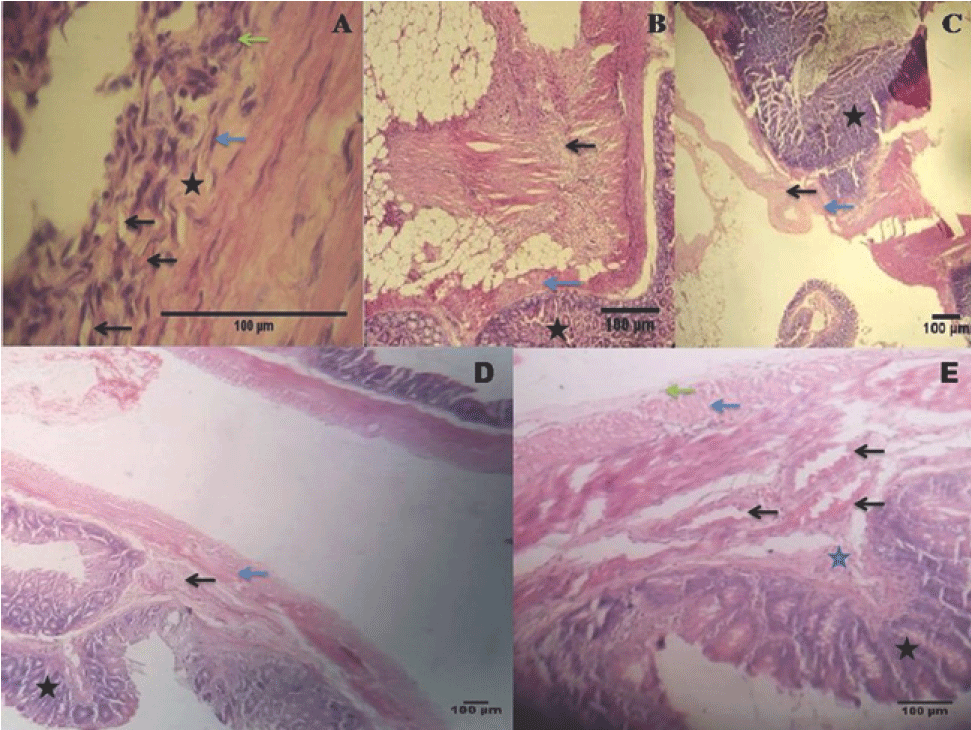
Images Obtained from the Histopathological Evaluation of Experimental Animals. A. Histopathological image representing a sample of tissue with adhesions in which large
areas of loose connective tissue and inflammatory infiltrate (star), with presence
of giant cells (green arrow), fibroblast (blue arrow) and mild vascular proliferation
(black arrow) were observed (Hematoxylin-eosin stain; original magnification X40).
B. Sample of tissue in which the intestinal mucosa (star), the muscularis externa (blue
arrow), areas of fibrosis and presence of infiltration of inflammatory cells (black
arrow) were observed (Hematoxylin-eosin stain; original magnification X10) C. Sample of tissue in which were evidenced: the intestinal mucosa (star), the muscularis
externa (blue arrow) and areas of loose connective tissue (black arrow). (Hematoxylin-eosin
stain; original magnification X5). D. Histo-logical image belonging to rat, in which no adhesions were observed in the
macroscopic evaluation. Highlighted areas include the intestinal mucosa (star), the
submucosa (black arrow) and the muscularis externa (blue arrow). (Hematoxylin-eosin
stain; original magnification X5). E. Histological image in which the intestinal mucosa (black star), the submucosa (blue
star) with blood vessels (black arrow), the muscularis externa (blue arrow) and the
serosa (green arrow), without inflammatory process, vascular proliferation and fibrosis.
(Hematoxylin-eosin stain; original magnification X10).
Likewise, a statistically significant difference was observed when the inflammation
and vascular proliferation were evaluated in both experimental groups, denoting p
values of p = 0.002 and p = 0.004, respectively.
Discussion
The objective of this study was to evaluate the effects of Meloxicam on the formation
of postoperative peritoneal adhesions in an experimental animal model, which consisted
of the serous abrasion of the rat colon. The results of the macroscopic evaluation
showed that the animals belonging to the group administered Meloxicam developed fewer
adhesions and had no adhesions in unmanipulated organs. As for the histological evaluation,
it was evidenced that the Meloxicam group under study developed a lower degree of
fibrosis, inflammation and vascular proliferation. In contrast, after peritoneal trauma
by serosal abrasion of the colon, all rats in the control group developed peritoneal
adhesions and greater degrees of severity than the rats given Meloxicam.
The trauma to the peritoneum triggers a cascade of events that begins with the disruption
of mast cells, which release vasoactive substances such as histamine that increase
vascular permeability.6 In addition, extravasation of a fibrinogen rich fluid occurs
from the injured surfaces. Simultaneously, an inflammatory response occurs, with migration
of inflammatory cells, release of cytokines and activation of the coagulation cascade.
Activation of the coagulation system results in the formation of thrombin, which is
necessary for the conversion of fibrinogen to fibrin. Since fibrinolysis is the key
determinant in the formation of adhesions. If this does not occur within 5 to 7 days
following peritoneal injury, the fibrin matrix persists and is gradually further organized
with collagen secreting fibroblasts.4
Fibroblasts and myofibroblasts secrete massive amounts of extra-cellular matrix molecules
including fibronectin, hyaluronic acid, glycosaminoglycans, and proteoglycans. This
process establishes a bridge between tissues within a few weeks. Further evidence
includes vascularization and deposits of collagen in this adhesion bridge formed between
the two tissues.9
The formation of peritoneal adhesions results from a complex cascade regulated by
different cellular and humoral factors. Among the cellular factors are the mesothelial
cells, different types of inflammatory cells and fibroblasts. The relationship of
these cells and their structural organization is regulated by cytokines, growth factors
and signaling molecules. It is widely accepted that in local tissue injury, ischemia,
the resulting inflammatory response, and the promotion of procoagulatory processes
such as antifibrinolytic reactions are essential for the formation of peritoneal adhesions.23
Peritoneal damage causes an inflammatory response, in which inflammatory cells release
cytokines, such as Tumor Necrosis Factor alpha (TNF-alpha), interleukin 1 and 6. These
cytokines induce the release of plasminogen activator inhibitor-1 and 2 (PAI-1 and
PAI-2) of mesothelial cells, which results in a reduction in Plasminogen activator
(Pas) activity. In this way, PAI inhibits fibrinolysis, and fibrin deposits are infiltrated
by granulocytes, monocytes and fibroblasts, followed by capillary growth, collagen
deposits and adhesion formation.24
Likewise, an increase in COX-2 expression has been shown in response to hypoxia in
normal peritoneum fibroblasts. However, COX-1 expression remains unchanged in adhesions
and fibroblasts under conditions of normoxia and hypoxia. It is hypothesized that
hypoxia leads to fibroblasts of the normal peritoneum to acquire a phenotype of adhesions
as a manifestation of the marked increase in the expression of COX-2. 25 Hypoxia induces
normal peritoneal fibroblasts to produce high levels of PGE-2 and COX-2, an effect
that can be prevented by inhibition of COX-2. Therefore, it is considered that COX-2
and its inhibitor may play a role in the postoperative regulation of tissue repair
and the development of adhesions.26, 27
VEGF is an angiogenic cytokine that participates in the adhesion formation process
through the formation of new vessels. It is implicated in early inflammatory responses,
tissue repair and remodeling through fibroblast function. It is also important to
facilitate fibrin rich matrix deposition, necessary for cell migration and proliferation.28, 29
Also, it has been suggested that laparotomy can stimulate the formation of adhesions
through a cellular process dependent on mast cells, an inflammatory process that is
independent of immediate degranulation. Mast cells are probably not responsible for
all locally released VEGF, because this cell produces cytokines that induce the influx
of other inflammatory cells that could produce VEGF at the site of the lesion.28, 29
It has been shown that dexamethasone, a steroidal anti-inflammatory, in combination
with sodium carboxymethylcellulose can prevent the formation of adhesions in a rat
adhesion-forming model, inhibiting the migration of inflammatory cells, further decreasing
the proliferation of fibroblasts.30
Likewise, the use of non-steroidal anti-inflammatory drugs, such as Diclofenac Sodium,
has been effective in decreasing adhesion formation in a model of anti-mesenteric
border lesion in the rat colon. In this same study, there was a decrease in the development
of edema, hyperemia, inflammation and fibrosis.31
Similarly, inhibition of COX-2 by the administration of parecoxib, celecoxib, rofecoxib
and nimesulide has been shown to decrease adhesion formation in animal models.32,33,34,35 Guvenal et al. associated the effects of nimesulide in its study to its anti-prostaglandin
activity and the reduction of the production of anti-angiogenic cytokines.34
However, in a study by Keskin et al., where Meloxicam and Dexketoprofen were evaluated
in a rat uterine horn surgical model, it was shown that Meloxicam decreased the development
of inflammation. However, despite a decrease in vascular proliferation and collagen
formation, there was no significant decrease in adhesion formation.36 In contrast,
in the present study Meloxicam was shown to decrease the formation of adhesions, the
development of fibrosis, vascular proliferation and inflammation. This may be due
to the fact that different techniques and experimental models were used in both studies.
In addition, in the same study, Meloxicam was administered 2 days before surgery and
5 days after surgery, whereas in this study meloxicam was given for 7 days after the
surgical procedure.
In an experimental model of periodontitis in rats, it was shown that after 14 days
of treatment with Meloxicam, there was a decrease in the expression of VEGF expression.37
Similarly, it has been shown that Meloxicam decreases VEGF levels in tumor tissues
from animal experimental models.38 In a model of ovarian hyperstimulation syndrome
in rats, the results suggest that Meloxicam affects the expression of VEGF in the
ovary.39
In addition to the inhibition of the inflammatory process involved in the formation
of adhesions in the Meloxicam group, there was also a significant decrease in the
development of vascular proliferation. The mechanism is most likely due to a decrease
in the production of VEGF by the cells involved in the process of adhesionogenesis,
such as mast cells.
Some limitations were evident in our study. The pathophysiological process of peritoneal
scarring was one of the limitations, because the mechanisms of adhesion formation
in human have not been studied in great detail to warrant a direct comparison to the
effects of meloxicam on adhesion formation in rats. The adverse and side effects of
Meloxicam was also not examined in the study at the dose (0.20 mg/kg/day) and duration
of treatment (7 days) used in the study. Despite the limitations of the study, meloxicam
was shown to decrease the formation of postoperative peritoneal adhesions in the experimental
model used.
In conclusion, Meloxicam, proved to be effective in the prevention of post-surgical
peritoneal adhesions induced in the animal model used. It is a promising finding,
based on the pathophysiological knowledge of inflammation, peritoneal healing and
the involvement of VEGF in the formation of peritoneal adhesions. Therefore, it is
proposed to continue the research of this drug in other models of experimentation,
while performing the quantification of inflammatory markers, cytokines in plasma and
peritoneal fluid. The final objective of future studies will be to understand the
effects, positive or negative, of Meloxicam in the formation of adhesions.
Acknowledgments
Special acknowledgments go to the School of Health Sciences, “Dr. Francisco Battistini
Casalta”, and the University of Oriente.
Conflict of Interest Statement & Funding
None of the authors shows any conflicts of interest during the investigation or publication
of this article.
Author Contributions
Conception and design the work/idea, Write de manuscript, Contribution of patients
or study material, Statistical advice: LAHV. Collect data / obtaining results: LAHV,
HF. Analysis and interpretation of data, Critical revision of the manuscript, Approval
of the final version: LAHV, HF, LC.
References
1. Bozdag Z, Gumus M, Arikanoglu Z, Ibiloglu I, Kaya S, Evliyaoglu O. Effect of intraperitoneal Thymoquinone on Postoperative Peritoneal Adhesions. Acta Chir Belg. 2015 (5); 115: 364–368.
2. De Clerq K, Sheltfhout C, Bracke M, De Wever O, Van Bockstal M, Ceelen W, et al. Genipin-crosslinked gelatin microspheres as a strategy to prevent postsurgical peritoneal
adhesions: In vitro and in vivo characterization. Bio-materials. 2016; 96: 33–46.
3. Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ. Laparoscopic Lysis of Adhesions. World J Surg. 2006; 30(4): 535–40.
4. Schnuriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 2011; 201(1): 111–121.
5. Dubcenco E, Assumpcao L, Dray X, Gabrielson KL, Ruben DS, Pipitone LJ, et al. Adhesion formation after peritoneoscopy with liver biopsy in a survival porcine model:
comparison of laparotomy, laparoscopy, and transgastric natural orifice transluminal
endoscopic surgery (NOTES). Endoscopy. 2009; 41(11): 971–978.
6. Practice Committee of American Society for Reproductive Medicine in collaboration
with Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery:
a committee opinion. Fertil Steril. 2013; 99(6): 1550–1555.
7. Rajab TK, Wallwiener M, Talukdar S, Kraemer B. Adhesion-Related Complications Are Common, But Rarely Discussed in Preoperative Consent:
A Multicenter Study. World J Surg. 2009; 33(4): 748–750.
8. ten Broek RP, Issa Y, van Santbrink EJ, Bouvy ND, Kruitwagen RF, Jeeker J, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ. 2013; 347: f5588.
9. Ward BC, Panitch A. Abdominal Adhesion: Current and Novel Therapies. J Surg Res. 2011; 165(1): 91–111.
10. Ahmad G, Duffy JM, Farquhar C, Vail A, Vandekerchove P, Watson A, et al. Barrier agents for adhesion prevention after gyneacological surgery (Review). Cochrane Database of Syst Rev. 2008;(2):CD000475.
11. Wallwiener M, Brucker S, Hierlemann H, Brochhausen C, Solomayer E, Wallwiener C. Innovative barriers for peritoneal adhesion prevention: liquid or solid? A rat uterine
horn model. Fertil Steril. 2006; 86(4 Suppl): 1266–1276.
12. Ayala M, Ramírez E, Quiroz J, Ortiz J, González B. [Role of alopurinol in peritoneal adherences when placing a polypropylene mesh: Experimental
study]. Cir Gen. 2013; 35(1): 16–19.
13. Fotiadis K, Filidou E, Arvanitidis K, Valatas V, Stavrou G, Basdanis G, et al. Intraperitoneal application of phospholipids for the prevention of postoperative adhesions:
a possible role of myofibroblasts. J Surg Res. 2015;197(2): 291–300.
14. Vázques CJ, Ortiz MM, Sánchez GJR, Reynoso VJ, Gutiérrez I, Gutiérrez C. [Decrease of angiogenesis with spironolactone and captopril and the effect on intraperitoneal
adherences]. Cir Gen. 2007; 29(4): 265–268. Spanish.
15. Maciver AH, McCall M, James Shapiro AM. Intra-abdominal adhesions: cellular mechanisms and strategies for prevention. Int J Surg. 2011; 9(8): 589–594.
16. Hoffmann NE, Siddiqui SA, Agarwal S, McKellar SH, Kurtz HJ, Gettman MT, et al. Choice of Hemostatic Agent Influences Adhesion Formation in a Rat Cecal Adhesion Model. J Surg Res. 2009; 155(1): 77–81.
17. Yetkin G, Uludag M, Citgez B, Karakoc S, Polat N, Kabukcuoglu F. Prevention of peritoneal adhesions by intraperitoneal administration of vitamin E
and human amniotic membrane. Int J Surg. 2009; 7(6): 561–565.
18. Koninckx P, Corona R, Timmerman D, Verguts J, Adamyan L. Peritoneal full-conditioning reduces postoperative adhesions and pain: a randomised
controlled trial in deep endometriosis surgery. J Ovarian Res. 2013; 6(1):90.
19. Huang LN, Yao XM. Inhibitory effect of Meloxicam on the cultured fibro-blasts from the excised pterygium. Int J Ophthalmol. 2008; 1(1): 48–51.
20. AVECAL (Venezuelan Association for the Science of Laboratory Animals). [Manual for the production and ethical use of laboratory animals]. Ministry of Popular Power for Science and Technology. Caracas. 2008. Spanish.
21. Mayagoitia-Gonzalez JC, Gudiño-Amezcua LM, Rivera-Barragan V, Mellado-Diaz AV, Díaz-Chavez EP. [Prevention of intestinal adhesions, through the addition of hyaluronic acid/carboxymethylcellulose
gel. Experimental model in rats]. Cir Cir. 2012; 80(2): 150–156.
22. Marentes Etienne JJ, Joya Cervera RE, Rodríguez Hernandez A, Díaz-Chavez EP. [Efficacy of a silicone composite mesh to reduce intra-abdominal adhesions in wistar
rats: A preliminary report]. Cir Gen. 2014; 36(4): 205–208. Spanish.
23. Brochhausen C, Schmitt VH, Planck CN, Rajab TK, Hollemann D, Tapprich C, et al. Current strategies and future perspectives for intraperitoneal adhesion prevention. J Gastrointest Surg. 2012; 16(6): 1256–1274.
24. Fredriksson F, Christofferson RH, Carlsson PO, Lilja HE. Locally increased concentrations of inflammatory cytokines in an experimental intrabdominal
adhesion model. J Pediatr Surg. 2014; 49(10): 1480–1484.
25. Braun KM, Diamond MP. The Biology of Adhesion Formation in the Peritoneal Cavity. Semin Pediatr Surg. 2014; 23(6): 336–343.
26. Saed GM, Munkarah AR, Diamond MP. Cycloxygenase-2 is expressed in human fibroblasts isolated from intraperitoneal adhesions
but not from normal peritoneal tissues. Fertil Steril. 2003; 79(6): 1404–1408.
27. Saed GM, Munkarah AR, Abu-Soud HM, Diamond MP. Hypoxia upregulates cycloxygenase-2 and prostaglandin E2 levels in human peritoneal
fibroblasts. Fertil Steril. 2005; 83 Suppl 1: 1216–1219.
28. Cahill RA, Wang JH, Soohkai S, Redmond HP. Mast cells facilitate local VEGF release as an early event in the pathogenesis of
postoperative peritoneal adhesions. Surgery. 2006; 140(1): 108–112.
29. Cahill RA, Redmond HP. Cytokine orchestration in post-operative peritoneal adhesion formation. World J Gastroenterol. 2008; 14(31): 4861–4866.
30. Du XH, Liu JQ, Xin K, Liu GH. Dexamethasone and sodium carboxymethyl cellulose prevent postoperative intraperitoneal
adhesions in rats. Braz J Med Biol Res. 2015; 48(4): 344–348.
31. Allahverdi TD, Allaverdi E, Yayla S, Deprem T, Merhan O, Vural S. The Comparison of the Effects of Ellagic Acid and Diclofenac Sodium on Intra-Abdominal
Adhesion: An In Vivo Study in the Rat Model. Int Surg. 2014; 99(5): 543–550.
32. Arung W, Jehaes F, Cheramy JP, Defraigne JO, Meurisse M, Honore P, et al. Effects of Parecoxib on The Prevention of Postoperative Peritoneal Adhesions in Rats. J Invest Surg. 2013; 26(6): 340–346.
33. Greene AK, Alwayn IP, Nose V, Flynn E, Sampson D, Zurakowski D, et al. Prevention of Intra-abdominal Adhesions Using the Antiangiogenic COX-2 inhibitor Celecoxib. Ann Surg. 2005; 242(1): 140–146.
34. Guvenal T, Cetin A, Ozdemir H, Yanar O, Kaya T. Prevention of postoperative adhesión formation in rat uterine horn model by nimesulide:
a selective COX-2 inhibitor. Hum Reprod. 2001;16(8): 1732–1735.
35. Guvenal T, Yanar O, Timuroglu Y, Cetin M, Cetin A. Effects of selective and non-selective cyclooxygenase (COX) inhibitors on postoperative
adhesión formation in a rat uterine horn model. Clin Exp Obstet Gynecol. 2010; 37(1): 49–52.
36. Keskin HL, Akkus SM, Sirens YS, Ustuner I, Keles H, Ide T, et al. Comparison of the Effects of Meloxicam and Dexketoprofen on Postoperative Adhesion
Formation in a Rat Uterine Horn Surgical Model. J Minim Invasive Gynecol. 2013; 20(2): 185–191.
37. Oliveira TM, Sakai VT, Machado MA, Dionísio TJ, Cestari TM, Taga R, et al. COX-2 Inhibition Decreases VEGF Expression and Alveolar Bone loss During the Progression
of Experimental Periodontitis in Rats. J Periodontol. 2008; 79(6): 1062–1069.
38. Xin B, Yokoyama Y, Shigeto T, Futagami M, Mizunuma H. Inhibitory Effects of Meloxicam, a Selective Cyclooxygenase-2 inhibitor, and Ciglitazone,
a Peroxisome Proliferator-Activated Receptor Gamma Ligand, on the Growth of Human
Ovarian Cancers. Cancer. 2007; 110(4): 791–800.
39. Quintana R, Kopcow L, Marconi G, Young E, Yovanovich C, Paz DA. Inhibition of cyclooxygenase-2 (COX-2) by meloxicam decreases the incidence of ovarian
hyperstimulation syndrome in a rat model. Fertil Steril. 2008; 90 (4 Suppl): 1511–1516.
Luis Alfredo Hernandez Villarroel, 1 University of Oriente
Henry Fernandez, 1 University of Oriente
Luisa Cesin, 1 University of Oriente
Mihnea-Alexandru Găman, Editor
About the Author: Luis Hernández is a doctor of medicine, recently graduated from the University of
Oriente. He is winner of the second place in the international competition of research
works in the XXVIII International Scientific Congress of the Latin American Federation
of Scientific Societies of Medical Students and winner of the first place in clinical
cases and the third place in research works in the III Congress of Students of Medicine
of the University of the Andes and Regional Course Zone C of the Latin American Federation
of Scientific Societies of Medical Students.
Correspondence Luis Alfredo Hernandez Villarroel, Email: luisvango@hotmail.com
Cite as: Hernandez-Villarroel LA, Fernandez H, Cesin L. Meloxicam Decreases the Formation of Peritoneal Adhesions in an Experimental Surgical Model in Rats. Int J Med Students. 2017 Jan-Apr;5(1):6-13.
Copyright © 2017 Luis Alfredo Hernandez Villarroel, Henry Fernandez, Luisa Cesin
International Journal of Medical Students, VOLUME 5, NUMBER 1, April 2017




