Reviews
Integrating Tendinous Pathophysiology Into Rotator Cuff Tears And Greater Trochanteric
Pain Syndrome: A Narrative Review
Joshua R. Poole1, Erin Alaia2, Robert J. Meislin3
doi: http://dx.doi.org/ijms.2024.2023
Volume 12, Number 3: 303-310
Received 17 03 2023;
Rev-request 12 06 2023;
Rev-request 14 12 2023;
Rev-recd 03 07 2023;
Rev-recd 26 12 2023;
Accepted 08 06 2024
ABSTRACT
This narrative review aims to use the similarities between the shoulder and hip joints
to better understand why rotator cuff (RC) tendinopathy and hip abductor tendinopathy
occur and inform about diagnosis and treatment of both orthopedic complaints. A search
of the literature was done using Google Scholar and Pubmed and initially followed
a systematic review protocol, but the nature of the topic, current literature and
data necessitated a narrative review. Reports that discussed pathomechanics of RC
and gluteal tendinopathy individually, together and with other muscles groups were
reviewed. It was found that the methods measuring and describing the processes of
tendinopathy differ significantly, for each individual joint and between all joints.
A review of a large body of quantifiable measures and theoretical ideas regarding
tendinopathy was performed to address this lack of consensus in current literature.
Initial literature yielded 74 articles. After review, only 43 articles were used from
a broad range of approaches and methodologies. The review found a body of evidence
suggesting that fibrocartilage overgrowth and compressive forces over bony structures
cause tendonous pathology of the RC and hip abductor tendons. These findings support
the idea that tendinopathy is often caused by intrinsic factors rather than the traditional
view of external factors. Earlier treatment and improved outcomes without surgery
are possible using current imaging technology to identify these intrinsic factors
that affect tendinous properties.
Introduction
The similarities between the musculature of the shoulder joint and the hip joint have
long been acknowledged. The tendons surrounding the hip joint are often referred to
as the “rotator cuff of the hip”.1–4 This comparison is fitting because of how similar the joints are in anatomy, pathology,
and treatment. The muscles that of the rotator cuff (RC) of the shoulder and hip abductors
that have drawn the most comparison include the subscapularis, supraspinatus, infraspinatus,
teres minor, gluteus medius and gluteus minimus. Current literature is replete with
connections between these muscle groups.
The RC has been more thoroughly studied than the hip abductors primarily due to the
prevalence of RC injury. Tears of the hip abductors are often discussed in terms of
findings in RC literature. Treatments for hip abductor injury and lateral hip pain
are even discussed in terms of effectiveness in the RC rather than the gluteus medius
or minimus.2 Lateral hip pain affects 1.8 out of 1000 patients annually in the United states5. For many years, pain of the lateral hip has been poorly understood resulting in
a diagnosis of trochanteric bursitis without further clinical investigation.2 Recent research suggests that the differential is far more broad. Greater trochanteric
pain syndrome (GTPS) has gained traction as one of the newly considered diagnosis
for lateral hip pain and in some studies is considered more prevalent than Trochanteric
bursitis.2,6,7 In one particular study, MRI was used to confirm diagnosis of trochanteric bursitis
in 24 female patients. It found that 45.8% of patients had gluteus medius tears and
62.5% had isolated gluteus medius tendinopathy with no findings of trochanteric bursitis8. As a result of many studies like this, trochanteric bursitis is no longer accepted
as the principal cause of lateral hip. It is now just a part of the broad differential
of lateral hip pain8,9. Causes of lateral hip pain are often injuries that lead to hip abductor tears. Hip
abductor tears are due to degenerative wear that occurs commonly in adults over 40
years old, more commonly in women, and rarely occur because of trauma.5,10 In exploring the role of GTPS in hip abductor pathophysiology we can start to understand
its similarity to RC tendinopathy.
In orthopedics, pain of the RC and limited shoulder range of motion are among the
most common complaints and the more heavily studied conditions. It is currently considered
the third most common musculoskeletal complaints in the united states.11,12 RC cuff based complaints have a lifetime prevalence of 67% in the Unites States and
are also highly age dependent with a global prevalence between 5–10% in patients 20
years old or younger and above 60% in those older than 80 years old (77). Despite
its prevalence its pathophysiology is still not well defined. Dr. Charles Neer first
described impingement of the supraspinatus muscles in 1972. He proposed that extrinsic
acromial impingement was the principal cause of the symptoms experienced so commonly
and recommended acromioplasty and coracoacromial ligament release as treatment.13,14 At the time, his idea was well accepted and followed but has since been challenged.
In fact, many recent studies suggest that rotator cuff tendinopathy and eventual tears
have very little to do with acromial impingement but rather due to intrinsic impingement
and apoptosis.13–18 Extrinsic acromial impingement as the cause of RC tendinopathy is further questioned
by a 2012 report from the American Academy of Orthopedic Surgeons that declared “routine
acromioplasty is not required at the time of rotator cuff repair”.19
Describing RC injury or gluteus medius/minimus tear in terms of impingement or trochanteric
bursitis limits the thinking of researchers and physicians alike.14 Arguments and evidence in support of the prevalence of acromial impingement and trochanteric
bursitis are still present but the frequency and impact of these arguments has since
diminished. The original insights have led to new theories and ideas surrounding tendinopathy
and tears of the hip abductors and RC. Researching these gaps in current literature
is important to providing the highest quality of care based upon the most accurate
understanding of these complaints. The aim of this paper is to review current literature
on the anatomy, pathology, and biomechanics of each muscle group to understand the
cause of both sets of symptoms more fully. Elaborating on the causes of these orthopedic
complains can lead to better treatment and as a result better patient outcomes.
Methods
A comprehensive search was conducted using two electronic databases, Google Scholar
and PubMed, to identify articles related to the pathomechanics of RC and gluteal tendinopathy.
The search terms included keywords such as “Rotator Cuff,” “Gluteal Tendinopathy,”
“Greater Trochanteric Pain Syndrome,” and “Hip Abductor Tendons.” Articles that discussed
the pathomechanics of RC and gluteal tendinopathy individually, together, and with
other muscle groups were included in the review. The initial search results were screened
by title and abstract for relevance and yielded 74 articles by two authors. These
articles were then reviewed in detail with a focus on identifying the causes of tendonous
pathology in the RC and hip abductor tendons. After a thorough screening process,
43 articles were used to provide the insights found in this review. These articles
were from a broad range of approaches, methodologies and demographics, including biomechanical
studies, clinical trials, and case reports. When necessary, articles were critically
evaluated to determine the quality of evidence presented and to extract relevant data.
Not all papers were used for statistical reasons but rather for insight and theories
in order to collect a broad range of ideas to consider in this narrative review. The
nature of the topic demanded this approach given its limited current research. Scale
for the Assessment of Narrative Review Articles (SANRA) was used to guide this review.
Results
Anatomical Comparison of the Hip and Shoulder Joint Musculature
The supraspinatus originates from the supraspinous fossa. It crosses underneath the
acromion, passes over the glenoid and bicipital groove to attach to the greater tuberosity
on the anterior lateral side of the humerus deep to the deltoid. It is a primary initiator
of abduction of the shoulder. Gluteus minimus originates from the area of the ilium
between the anterior and inferior gluteal lines. Gluteus medius originates from the
area of the ilium between the anterior and inferior gluteal lines. The gluteus medius
inserts into the lateral and superior aspect of the greater trochanter. Gluteus minimus
inserts onto the anterolateral aspect of the greater trochanter located deep to the
Iliotibial band band/tensor fascia lata. Both muscles function to abduct and medially
rotate the hip are the primary muscles of concern for tendinous degeneration. Both
tendons course posterior medially to inferior lateral and attach just millimeters
after taking a sharp turn over bony prominence. This is one of the major contributors
to the pathology of these muscles Table 1.
Table 1.
Comparing Function of Shoulder and Hip Joint Musculature.
| Hip |
Rotator Cuff |
Function |
| Gluteus medius/Gluteus minimius |
Supraspinatus |
Abduction/Stabilizer |
| Iliopsoas* |
Subscapularis |
Internal rotator |
| Piriformis* |
Infraspinatus/Teres minor |
External rotator |
| Rectus femoris |
Long head of biceps brachii |
Crosses over the anterior portion of both joint of the limb** |
| Iliotibial band/Tensor fascia lata |
deltoid |
Overlies the bony prominance |
Legend: Shoulder and hip joints have muscles that are identical in function, directionality,
and contribution to pathology of the joint.
*One of many,
**In the lower extremity this includes the knee and the hip, in the upper extremity
this includes the elbow and shoulder.
Infraspinatus and teres minor attach proximally to the infraspinous fossa. The infraspinatus
courses superior to medial and the teres minor courses laterally. The infraspinatus
inserts on the greater tuberosity inferior and posterior to the supraspinatus insertion.
It shares fibrous insertion with the supraspinatus. For this reason, the infraspinatus
tendon is also of concern for RC based injury. The teres minor inserts on the greater
tubercle just inferior to the infraspinatus insertion. The piriformis originates from
the pelvic surface of the sacrum and inserts on the superior border of the greater
trochanter. All three of these muscles function to externally rotate their respective
joints.
The subscapularis originates at the subscapular fossa and inserts onto the lesser
tuberosity of the humerus. The Iliopsoas is composed of the psoas and the iliacus
which originate at the transverse process and vertebral bodies of T12-L5 and the iliac
crest respectively. These muscles join as a common tendon that attaches onto the lesser
trochanter. Both muscles originate posterior from their insertion. All these muscles
function to internally rotate the joint.
The long head of the biceps brachii originates at the supraglenoid tubercle and inserts
at the radial tuberosity. Its proximal tendon courses through the bicipital groove
as it crosses the glenohumeral joint. It most prominently flexes the arm but also
functions to flex the shoulder. The rectus femoris originates at the anterior inferior
iliac spine and inserts onto the tibial tuberosity via the patellar tendon. This muscle
primarily flexes the hip and extends the knee. Both muscles are unique because they
course anteriorly across two joints and influence motion of both.
The deltoid originates from the distal third of the clavicle, the acromion and the
spine of the scapula. All three parts of the muscle converge to form the deltoid tendon
and insert on the deltoid tuberosity of the humerus. The tensor fascia lata originates
at the anterior superior iliac spine and anterior one third of the outer lip of the
iliac crest. Parts of the gluteus maximus fuse with the tensor fascia lata to form
the iliotibial band wphich inserts on the lateral aspect of the tibia via the iliotibial
tract. These muscles are the most superficial and lateral of their respective joints
and serve as a means of compression over the RC and hip abductors respectively. This
results in higher compressive forces over the tendons.
Biomechanics
Tendons are naturally developed to withstand large tensile loads but there is little
known about their ability to withstand compressive forces. Tendons are so strong in
resisting tears from tensile loads that tears occur at the muscle tendon junction
before the bony insertion under extreme testing conditions.20 The supraspinatus and the gluteus medius tendons are wrapped over the greater tuberosity
and the greater trochanter respectively until attaching to their insertions. To contract,
both muscles must transmit forces nonlinearly from attachment to insertion and exert
force onto the joint. This vector relationship is not uncommon anatomically but what
sets these tendons apart is the extreme change in direction that occurs just before
insertion and the large forces exerted through muscles. Compressive forces alone do
not result in pathology. This is because of the bursa that is between the bone and
tendon. Rather, compressive forces can be a catalyst when combined with excessive
tensile forces.
The nature of the gluteus medius tendon differs based upon insertion location. The
anterolateral insertion is identified as the thinnest portion and the most prone to
injury and tendinopathy.1,9,21–24 Gluteus medius tears are mostly degenerative in nature, starting as undersurface
tears at the anterior border of the lateral facet footprint and progressing posteriorly
to become full-thickness tears.25 The supraspinatus tendon is also split into anterior and posterior portions. The
anterior portion has a higher modulus of elasticity and still more prone to pathology.26 Tears at both joints primarily occur on the undersurface of the tendons.6 The tendinous tissue along the bony prominence is stretched less compared to the
more superficial side during contraction and therefore experiences less tensile load
but experiences a greater compressive force along the bony prominence. Differences
in tensile load along cross-sectional areas of the tendon is often referred to as
stress shielding.10,27,28 This is further intensified by increased iltiotibial band tightness exerting that
exerts pressure onto the greater trochanter over the gluteal tendons. The same scenario
is seen with acromial pressure on the tendon of the RC. Hyperadducted positions are
implicated in the pathology of both joints because of the increased force down onto
bone when abduction is performed exiting hyperadducted positions. Abducting from an
acute angle of the tendon over the bone produces more downward force than in a larger
angle over the bone.10,29-31 Due to these differences in the nature of the forces exerted on the tissue they take
on different morphology within the tendon. This is the main thought as the leading
cause for these tendinopathies.20,,27,30-34 One of the major issues in current literature is whether these challenges are pathological
or adaptive to these tendons.
Metaplastic Changes in Fibrocartilage
According to a review done by Cook et al. and findings in Benjamin et al., tendinous
tissue that inserts onto the bony prominences are considered fibrocartilage.27,33 Tendons under influence of compressive forces undergo metaplasia consisting of a
transition from type I collagen to type II collagen, large proteoglycan aggregates,
and neurovascular infiltration. All of these changes lead to tissue with increased
capacity to withstand compressive forces. These metaplastic changes present new questions
such as whether these changes are natural to all tendons in response to compression
or are they specific to certain tendons and do they ultimately have a negative impact.
The review presented by Cook et al. reports these changes as native to the tissue.
Rather than being a pathological response, fibrocartilage is just a subset of tendons
specific to areas under compression. The principal theory proffered by Cook et al.
to explain the damage that leads to tendinopathy is that compressive forces lead to
reduced water content of the tendon which is essential to its ability to withstand
compressive forces.27 Grigg et al. shared research showing decreased tendon thickness in tendinopathy of
the Achilles tendon that supports this idea.35 This research is a good foundation for supporting this theory but will need further
application to the gluteus medius and rotator cuff.
Benjamin et al. support the idea that this form of tissue is native to “wrap around
tendons”.33 This suggests that these metaplastic changes occur strictly in wrap around tendons.
Regardless of this, they still question whether this process is pathological or adaptive
in nature.33,34 Another important observation made by Cook et al. is that the bony prominence that
the Achilles tendon rubs on is in close proximity to the tendinous attachment to the
calcaneus.27 This is of concern because the tendon is undergoing a transition from normal tendon
to that which attaches to the bone with fundamentally different types of tissue. They
briefly describe how the proximity of these two zones and increased fibrocartilage
metaplasia causes instability and tearing of the tendon. This expansion of fibrocartilage
outside of its normal zone is supported more previous reports because tears occur
primarily in the tendon in proximity to the enthesis, this insight will be elaborated
on later in this review.20,36
Continuum of Tendinous Pathology
Cellular and extracellular intratendonal differences have been investigated since
1978 when Gillard et al. found that tendons adapt to compressive and tensile loads.37 To prove this they surgically detached tendon from over a bony prominence to an insertion
with a direct course and observed a gradual loss of the fibrocartilage tissue. Afterwards
they reattached the tissue and saw an increase in fibrocartilage. This is evidence
of an adaptive measure by tendons to compensate for compressive forces but is very
outdated and may need reevaluation. Even the model of tendon morphology between normal,
fibrocartilage and pathology used by Cook et al. is from a paper written in 1996.27
As in most physiological processes, tendinopathy is considered a process that fluctuates
along a continuum of presentations based upon more recent models.36,38 Cook et al. presents tendinopathy in three stages and the initial stage of reactive
tendinopathy fits those pathological stages elaborated on already.38 Namely, compression leading to a change in composition of the tissue with higher
type II collagen and increased proteoglycans. The second stage is tendon disrepair
which is characterized by greater matrix breakdown and hyperplasia with chondrocytes
and myofibroblasts resulting in increased protein production including proteoglycan
and collagen deposition causing decreased organization in the matrix. The last stage
is degenerative tendinopathy. This stage includes areas of cell death due to apoptosis
and trauma or tenocyte exhaustion. There is little capacity for reversibility of pathology
at this stage.
In comparison, Macdonald et al. argue that the first stage of tendinopathy is not
a characteristic of adaptation but rather a pathological reaction to acute overload
or stress.15 Cook et al. describe this process as acute and easily reversible which implies it
to be a normal response to new stimuli within the tendon. They even call it a response
to compression but do not consider it in the context of wrap around tendons like the
RC of the gluteus medius.38
In a follow up report, Cook et al. suggest that all models of tendinopathy can be
divided into three categories.34 The category most in line with pathology of the RC and gluteus medius is the “tendon
cell response” model. It views tendinopathy as a direct result of tenocyte activation in response to compression.
It displays the exact morphology of those mentioned in development of fibrocartilage
in wrap around tendons. The problem they find is knowing when these changes go from
healthy adaptation to damaging pathology. They cite a study using the Achilles tendon
in Australian football players that shows this adaptation as a healthy part of increased
tendon load and use but the study also showed 3 of the 18 of the participants progressed
to a painful tendinopathy which complicated their conclusion of normal adaptation.39
Tendon Cell Response Model in the RC and Gluteus Medius
Direct evidence of the tendon cell response model in the RC and gluteus tendons is
minimal. Almekinders et al. share evidence about the gluteal tendon being thinner
in pathological states but does not comment on its compositional makeup.20 Allison et al. elaborates on muscle atrophy and weakening in gluteal tendons but
they are unsure if the atrophy is a result of the tendinopathy or if the atrophy comes
before the tendinopathy.31 Another model mentioned by Cook at al. explains tendinopathy as part of a lack of
electrical stimulation from chronic damage that is correlated with atrophy of muscle.
This model is not investigated in a particular muscle, but it is broadly applied to
all muscle tendinopathy, so knowing how this may apply to the RC or gluteal tendons
is conjectural. This draws into question whether the tendinopathy indirectly results
in reduced muscle function. Allison et al. also consider disuse atrophy from patients
involuntarily reducing use of the muscle plays a role in causing the atrophy of muscle.31
Strong evidence for compressive forces being the principal cause of gluteal tendinopathy
is supported by the findings of a study investigating why females experience a much
higher degree of gluteus medius tears.29 They found that on average females have a much lower femoral neck shaft angle, giving
them a slight coxa vara compared to males. This causes a more acute angle over which
the gluteus medius passes over the greater trochanter which translates to greater
compressive forces over the bony prominence.10,29-31 Evidence suggests that this is the cause of higher rates of gluteus medius tears
in adult females. In addition, repetitive movements into and out of hyper adduction
are implicated in worse outcomes and symptoms for gluteal pathology. Contraction of
the gluteal muscles in hyperadducted positions would maximize the force of the tendon
onto the bone due to a lower angle of contraction between insertion and attachment
as stated earlier in this review.20,30,32,33
In a study done by Soslowsky et al. tensile and modulus testing was performed on rat
supraspinatus where they compared repetitive tensile loads used to simulate acromion
impingement and overuse stress on the tendon.40 They found that acromial impingement alone was not enough to induce tendinopathy
but required both tensile overuse and impingement to replicate rotator cuff pathology.
They also found that the thickness of the tendon and diameter of the tendon was significantly
lower and the modulus was significantly lower. This supports the theory regarding
dehydration of the tendon and is in line with lower modulus being a likely effect
of these combined forces. They did not consider the impact of compressive forces intrinsic
to the tendons as they considered them to be a non-physiological component of the
supraspinatus.
Perhaps the greatest insight as to why these tendons ultimately fail has been shown
from fibrocartilage metaplasia as expressed by Cook et al.27 They demonstrated that tenocyte overactivity extends outside of the margins of fibrocartilage
to zones of the tendon that are essential to sustaining tensile load and the transition
into enthesis. This type of change requires destruction of existing tissue and deposition
of new matrix and cellular metaplasia. The expansion of these zones is detrimental
to other essential functions of the tendons. Just as the expansion of the fibrocartilage
is necessary, the expansion of normal tendinous tissue becomes a demand of the tendon
as less normal tendon is present. These two essential tissue types and functions compete
and ultimately decrease the overall functionality of the tendon leading to damage.
The tendon is stuck between repair, metaplasia and proper function. Tissue consisting
of proteoglycan and type II collagen along the bony surface and normal tendinous tissue
proximal to the bony surface coexist within a microscopic section of tendon. This
continuum of change and adaption with a single tendon ultimately limits the tendon's
ability to function and repair as needed. Impaired function and repair leads to the
damage that is seen in the RC and hip abductors and eventual tears (Figure 1).
Figure 1.
Progression of Rotator Cuff and Hip Abductor Tendinopathy.
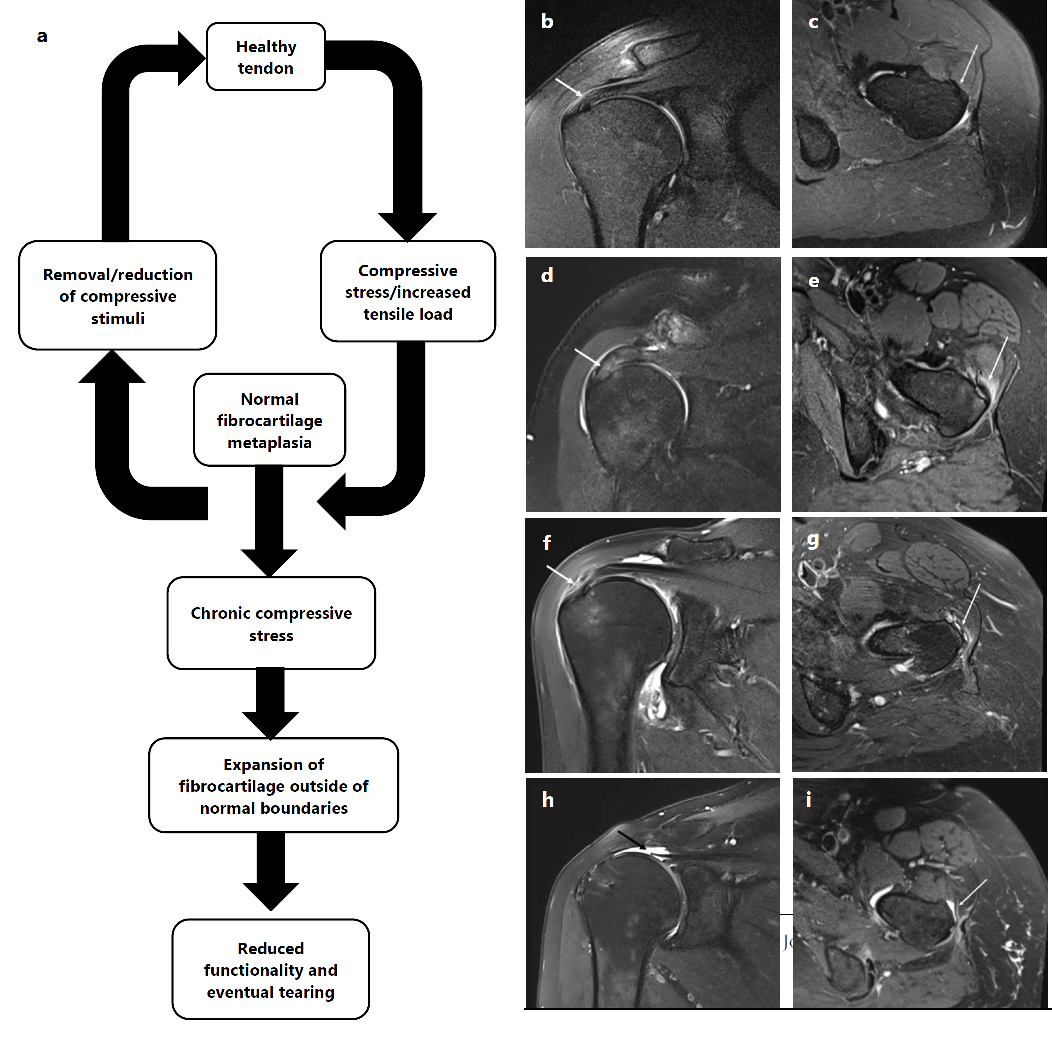
Legend: Side by side progression of tendinopathy of the RC and gluteus medius and minimus.
Injuries to both tendons occur on spectrum that is reversable at early stages but
may lead to chronic complications. (a) Healthy tendons that are exposed to compressive
and tensile stress compensate with fibrocartilaginous metaplasia. Metaplasia may be
sufficient to avoid damage and tearing. Alternatively, removal of the stimuli will
allow continued healthy function. Under chronic over exposure to compression and tensile
load, metaplasia disrupts proper function of the tendon and leads to pain and tearing.
(b) Coronal T2 fat suppressed image showing a normal supraspinatus tendon. (c) Axial
T2 fat suppressed image showing a normal gluteus minimus tendon. (d) Coronal T2 fat
suppressed image showing a supraspinatus tendinosis. (e) Axial T2 fat suppressed image
showing tendinosis of the gluteus minimus tendon. (f) Coronal T2 fat suppressed image
showing a severe supraspinatus tendinosis with a high-grade partial-thickness bursal
surface tear. (g) Axial T2 fat suppressed image showing tendinosis and high-grade
partial-thickness tearing of the glutes minimus tendon. (h) Coronal T2 fat suppressed
image showing a fill-thickness retracted re-tear of the supraspinatus tendon. (i)
Axial T2 fat suppressed image showing a complete tear of the gluteus minimus tendon
and underlying subgluteus minimums bursal fluid.
Discussion
This collection of evidence supports the idea that the source of damage to the tendons
is due to intrinsic adaptation. It is likely that these changes are both pathological
and adaptive. Fibrocartilage is an essential adaptation to compressional forces on
the tendon but can become pathological as demonstrated in Figure 1. In situations that decrease the muscle angle in females, over abducting or in overstressing
the tendon, fibrocartilage overgrows and ultimately leads to decreased functionality.
The need to investigate these findings is especially important to the RC and gluteal
tendon because these two tendons experience the greatest compressive forces, have
no definitive explanation for the cause of their pathology and are common complaints
among orthopedic patients. The gluteal tendon and RC are not a point of focus in the
research that defined these insights. This leaves a gap in the knowledge of these
two prevalent medical issues.
Clinical Implications
Patients who manifest an overgrowth of fibrocartilage could be treated at early stages
to reduce overgrowth and the damage that occurs in chronic stages of this pathology.
In detecting early morphological overgrowth, we can advise and treat the patient non-invasively
before the tendon is damaged enough to necessitate surgical intervention. Treatments
such as lifestyle and work modifications, physical therapy and pain management can
slow progression in chronic stages. The limiting factor is the ability to determine
the current morphological state of a tendon in vivo. The invasion of fibrocartilage
into adjacent zones would be a matter of micrometers in difference and is not currently
within the capabilities of modern imaging see Figure 1.
One alternative to this would be to measure the tensile modulus, compressive modulus
and thickness of the tendon over time. This relates to the idea of how the morphology
changes the water content and ability of the tendon to withstand certain forces.20,27,34–36,38,40 Tendon thickness would be indicative of certain stages of pathology. Early fibrocartilage
induces water retention and tendon thickening. Later tendinous damage would manifest
as a thinner tendon as water is poorly retained from over compression and damage.
Ability to withstand tensile load would decrease as normal tendon is broken down in
favor of fibrocartilage. This would manifest as a reduced tensile modulus.
Shear-wave elastography (SWE) and other ultrasonographic techniques are used currently
to measure these very parameters in tendons.41–45 SWE is widely available but rarely used in clinical practice. These methods of imaging
enable physicians to avoid invasive and expensive imaging in favor of measures that
tell us about the morphological state of the tissue. The principal measure that is
relevant to tendinopathy is tendon shear elastic modulus otherwise known as stiffness.
The higher stiffness in the tendon, the less metaplastic change has occurred in the
tendon. Dirrichs et al. used intraindividual and interindividual comparisons of tendinopathies
to compare SWE, ultrasound and power doppler.41 This study showed that SWE significantly improved the identification of painful tendinopathy
across a wide age group 20–71years old and different tendons including Achilles, patellar
and humeral epicondylar tendons.
Zhang et al. compared the patellar tendon stiffness and cross-sectional area of sedentary
and active 18–35 year old men.45 They found a significant decrease in stiffness and increase in cross-sectional area
in those who were more active and putting increased stress on their tendons. The authors
showed that the evaluated tendon demonstrated a normal response to tensile load. Taking
this in light of findings in the healthy active population we can correlate this with
a healthy metaplastic change in the tendons. The findings of Dirrichs et al.41 and Zhang et al.45 in some ways contradict each other but also support each other. The next step for
this diagnostic tool is to use cross sectional area, stiffness and patient presentation
can be used to create diagnostic criteria.
The next logical question to be addressed is whether these cross-sectional area and
stiffness parameters lead to tendinopathy impacting RC and gluteal tendinopathy treatment.
Performing experiments to correlate the tendon morphology to physical parameters and
matching them to stages of pathology within patients could potentially change the
patient experience and reduce the need for surgery in patients in the long term.
Limitations
This review is limited due to the nature of the topics and current literature available
for comparison and analysis that necessitated a narrative review. It relies primarily
on a collection of theories, ideas and anatomical understanding to build its argument.
This can be a foundation for a discussion that can lead to many other research opportunities
to test these theories spark innovation in orthopedic care of Hip tendinopathy and
shoulder tendinopathy.
Conclusion
Theories on the root cause of RC and hip abductor tendinous degeneration have evolved
over time. The anatomy, biomechanics and pathophysiology of these tendons is so similar
that we can use them to further our understanding. In doing so is it apparent that
increased tensile load and compression over bony prominences lead to metaplastic changes
within the tendon. These changes are natural adaptive responses that become pathological
with chronic overload of the tendon. The metaplastic changes decrease the capabilities
of the joints to function normally and lead to tearing of the tendon. This process
points to intrinsic factors of the tendons as being the principal cause of these complaints
as opposed to extrinsic factors. These intrinsic factors can be measured using modern
SWE techniques. In understanding and identifying early signs of degeneration that
are intrinsic to RC and hip abductor tendons that were previously unknown it may be
possible to slow or even stop the progression of tendon degeneration before a tear
becomes evident.
Summary – Accelerating Translation
This study explores the similarities between the muscles in the shoulder and hip joints,
and how this can provide a better understanding of the cellular and mechanical problems
associated with Greater Trochanteric Pain Syndrome and Rotator Cuff Tendinopathy.
This is a literature review and analysis of relevant insights and connections that
were found applicable to the aims of this project. By comparing these two conditions
along with current theories regarding of tendinopathy it is suspected that factors
within the tendons themselves, rather than external factors, lead to pain and tearing.
Shear Wave Elastography, a non-invasive ultrasound technique, can measure these intrinsic
factors and can be used to test these theories. The study suggests that earlier and
more affordable diagnosis and treatment of tendinopathy could be achieved by testing
the insights of this review.
Reference
1. Lequesne M. From “periarthritis” to hip “rotator cuff” tears. Trochanteric tendinobursitis. Joint Bone Spine. 2006;73(4):344–348.
2. Ho GWK, Howard TM. Greater trochanteric pain syndrome: more than bursitis and iliotibial tract friction. Curr Sports Med Rep. 2012;11(5):232–238.
3. Zhu MF, Musson DS, Cornish J, Young SW, Munro JT. Hip abductor tendon tears: where are we now?. Hip Int. 2020;30(5):500–512.
4. Bunker TD, Esler CN, Leach WJ. Rotator-cuff tear of the hip. J Bone Joint Surg Br. 1997;79(4):618–620.
5. Varacallo M, El Bitar Y, Mair SD. Rotator Cuff Tendonitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
6. Domb BG, Nasser RM, Botser IB. Partial-thickness tears of the gluteus medius: rationale and technique for trans-tendinous
endoscopic repair. Arthroscopy. 2010;26(12):1697–1705.
7. Dwek JR, Pfirrmann CW, Stanley AJ, Pathria MN, Chung CB. MR imaging of the hip abductors: normal anatomy and commonly encountered pathology
at the greater trochanter. Magn Reson Imaging Clin N Am. 2005;13(4):691–704.
8. Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings
in patients with greater trochanteric pain syndrome. Arthritis Rheum. 2001;44(9):2138–2145.
9. Connell DA, Bass C, Sykes CA, Young D, Edwards E. Sonographic evaluation of gluteus medius and minimus tendinopathy. Eur Radiol. 2003;13(6):1339–1347.
10. Grimaldi A, Fearon A. Gluteal tendinopathy: integrating pathomechanics and clinical features in its management. J Orthop Sports Phys Ther. 2015;45(11):910–922.
11. Syed MA, Azim SR, Baig M. Frequency of orthopedic problems among patients attending an orthopedic outpatient
department: a retrospective analysis of 23,495 cases. Ann Saudi Med. 2019;39(3):172–177.
12. Seidman A, Taqi M, Varacallo M. Trochanteric Bursitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
13. Neer CS II. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary
report. J Bone Joint Surg Am. 1972;54(1):41–50.
14. McFarland EG, Maffulli N, Del Buono A, Murrell GA, Garzon-Muvdi J, Petersen SA. Impingement is not impingement: the case for calling it “rotator cuff disease.”. Muscles Ligaments Tendons J. 2013;3(3):196–200.
15. MacDonald P, McRae S, Leiter J, Mascarenhas R, Lapner P. Arthroscopic rotator cuff repair with and without acromioplasty in the treatment of
full-thickness rotator cuff tears: a multicenter, randomized controlled trial. J Bone Joint Surg Am. 2011;93(21):1953–1960.
16. Milano G, Grasso A, Salvatore M, Zarelli D, Deriu L, Fabbriciani C. Arthroscopic rotator cuff repair with and without subacromial decompression: a prospective
randomized study. Arthroscopy. 2007;23(1):81–88.
17. Henkus HE, de Witte PB, Nelissen RG, Brand R, van Arkel ER. Bursectomy compared with acromioplasty in the management of subacromial impingement
syndrome: a prospective randomized study. J Bone Joint Surg Br. 2009;91(4):504–510.
18. Shin SJ, Oh JH, Chung SW, Song MK. The efficacy of acromioplasty in the arthroscopic repair of small to medium-sized
rotator cuff tears without acromial spur: prospective comparative study. Arthroscopy. 2012;28(5):628–635.
19. Pedowitz RA, Yamaguchi K, Ahmad CS, Burks RT, Flatow EL, et al Optimizing the management of rotator cuff problems. J Am Acad Orthop Surg. 2011;19(6):368–379.
20. Almekinders LC, Weinhold PS, Maffulli N. Compression etiology in tendinopathy. Clin Sports Med. 2003;22(4):703–710.
21. Tsutsumi M, Nimura A, Akita K. The gluteus medius tendon and its insertion sites: an anatomical study with possible
implications for gluteus medius tears. J Bone Joint Surg Am. 2019;101(2):177–184.
22. Walsh MJ, Walton JR, Walsh NA. Surgical repair of the gluteal tendons. J Arthroplasty. 2011;26(8):1514–1519.
23. Cvitanic O, Henzie G, Skezas N, Lyons J, Minter J. MRI diagnosis of tears of the hip abductor tendons (gluteus medius and gluteus minimus). AJR Am J Roentgenol. 2004;182(1):137–143.
24. Makridis KG, Lequesne M, Bard H, Dijan P. Clinical and MRI results in 67 patients operated for gluteus medius and minimus tendon
tears with a median follow-up of 4.6 years. Orthop Traumatol Surg Res. 2014;100(8):849–853.
25. Robertson WJ, Gardner MJ, Barker JU, Boraiah S, Lorich DG, Kelly BT. Anatomy and dimensions of the gluteus medius tendon insertion. Arthroscopy. 2008;24(2):130–136.
26. Huegel J, Williams AA, Soslowsky LJ. Rotator cuff biology and biomechanics: a review of normal and pathological conditions. Curr Rheumatol Rep. 2015;17(1):476.
27. Cook JL, Purdam CR. Is compressive load a factor in the development of tendinopathy?. Br J Sports Med. 2012;46(3):163–168.
28. Orchard J, Cook JL, Halpin N. Stress-shielding as a cause of insertional tendinopathy: the operative technique of
limited adductor tenotomy supports this theory. J Sci Med Sport. 2004;7(4):424–428.
29. Woyski D, Olinger A, Wright B. Smaller insertion area and inefficient mechanics of the gluteus medius in females. Surg Radiol Anat. 2013;35(8):713–719.
30. Birnbaum K, Siebert CH, Pandorf T, Schwenker C, Prescher A, Niethard FU. Anatomical and biomechanical investigations of the iliotibial tract. Surg Radiol Anat. 2004;26(6):433–446.
31. Allison K, Vicenzino B, Wrigley TV, Grimaldi A, Hodges PW, Bennell KL. Hip abductor muscle weakness in individuals with gluteal tendinopathy. Med Sci Sports Exerc. 2016;48(3):346–352.
32. Birnbaum K, Schwenker C, Hohmann D, Schrempf A, Prescher A, Bullmann V. Finite element model of the proximal femur under consideration of the hip centralizing
forces of the iliotibial tract. Clin Biomech (Bristol, Avon). 2011;26(1):58–64.
33. Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments—an adaptation to compressive load. J Anat. 1998;193(Pt 4):481–494.
34. Cook JL, Rio E, Purdam CR, Docking SI. Revisiting the continuum model of tendon pathology: what is its merit in clinical
practice and research?. Br J Sports Med. 2016;50(19):1187–1191.
35. Grigg NL, Wearing SC, Smeathers JE. Eccentric calf muscle exercise produces a greater acute reduction in Achilles tendon
thickness than concentric exercise. Br J Sports Med. 2009;43(4):280–283.
36. Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth
factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56(3):871–881.
37. Gillard GC, Reilly HC, Bell-Booth PG, Flint MH. The influence of mechanical forces on the glycosaminoglycan content of the rabbit
flexor digitorum profundus tendon. Connect Tissue Res. 1979;7(1):37–46.
38. Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation
of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–416.
39. van Schie HT, de Vos RJ, de Jonge S, Bakker EM, Heijboer MP, Verhaar JA, Tol JL. Ultrasonographic tissue characterisation of human Achilles tendons: quantification
of tendon structure through a novel non-invasive approach. Br J Sports Med. 2010;44(16):1153–1159.
40. Soslowsky LJ, Thomopoulos S, Esmail A, Flanagan CL, Iannotti JP, Williamson JD, Carpenter JE. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30(8):1057–1063.
41. Dirrichs T, Quack V, Gatz M, Tingart M, Kuhl C, Schrading S. Shear wave elastography (SWE) for the evaluation of patients with tendinopathies. Acad Radiol. 2016;23(10):1204–1213.
42. Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, Gao L, Witte RS. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37(3):855–870.
43. Feng YN, Li YP, Liu CL, Zhang ZJ. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound
elastography and MyotonPRO. Sci Rep. 2018;8(1):17064.
44. Garra BS. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007;23(4):255–268.
45. Zhang ZJ, Ng GY, Fu SN. Effects of habitual loading on patellar tendon mechanical and morphological properties
in basketball and volleyball players. Eur J Appl Physiol. 2015;115(11):2263–2269.
Joshua R. Poole, 1 First-year Medical Student. Arizona College of Osteopathic Medicine/Midwestern University,
Glendale, USA.
Erin Alaia, 2 MD. Department of Radiology at NYU School of Medicine, New York, USA.
Robert J. Meislin, 3 MD. Department of Orthopedic Surgery at NYU School of Medicine, New York, USA.
About the Author: Joshua Poole is a third–year medical student in a four program Arizona College of
Osteopathic Medicine in Glendale, Arizona USA which is a four-year program. He has
prepared this manuscript as part of his Externship in Orthopedic Surgery at New York
University.
Correspondence: Joshua R. Poole. Address: Ocotillo Hall, 19555 59th Ave, Glendale, AZ 85308, USA.
Email: Joshua.poole@midwestern.edu
Editor: Francisco J. Bonilla-Escobar;
Student Editors: Tangmi Djabo Eric, Shuchi Abhishek Adrien & Malina Cernatescu;
Proofreader: Laeeqa Manji;
Layout Editor: Julian A. Zapata-Rios;
Process: Peer-reviewed
Cite as
Poole JR, Alaia E, Meislin RJ. Integrating Tendinous Pathophysiology Into Rotator
Cuff Tears And Greater Trochanteric Pain. Int J Med Stud. 2024 Jul-Sep;12(3):303-310.
Copyright © 2024 Joshua R. Poole, Erin Alaia, Robert J. Meislin
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 12, NUMBER 3, July 2024
