Original Articles
A Descriptive Analysis of the Use of Various Therapeutics in a Cohort of COVID-19
Patients and the Influence of Media Coverage
Alfred A. Mathew1*, Barbara Mensah2*, John C. Cravero2, David C. Moffatt2, Roshan Dongre3, Thao K. Giang2, Samantha C. Olvera2, Susan L.F. McLellan4, Corri B. Levine5
doi: http://dx.doi.org/ijms.2024.2125
Volume 12, Number 3: 259-266
Received 22 06 2023;
Rev-request 05 08 2023;
Rev-request 21 02 2024;
Rev-recd 03 11 2023;
Rev-recd 12 07 2024;
Accepted 14 07 2024
ABSTRACT
Background:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impacted the healthcare
system immensely throughout 2020–2022. Treatment practices varied in Texas, as guidelines
were in flux. As a result, a variety of therapeutics were used. Many verified medications
with scientific basis were trialed, while others were implemented despite a lack of
scientific consensus. This study aimed to identify how practice patterns to treat
and manage COVID-19 changed over time in a cohort of patients in the University of
Texas Medical Branch hospital system.
Methods:
Ninety participants with a COVID-19 diagnosis were included in the analysis for this
study. Data was collected by a retrospective chart review, and included medications
administered before and during current admission. Medications were categorized as:
antiviral, antibiotic, steroid, supplement, antibody, hydroxychloroquine, and others.
Results:
Differences in therapeutic use were identified based on hospitalization status (outpatient
or inpatient) and month admitted. The largest difference in the antiviral remdesivir
(78%), requiring intravenous administration for up to ten days. In the outpatient
setting, antibiotics, primarily azithromycin, were quite common. Additionally, month-to-month
variation in steroid use and antibiotic use was observed.
Conclusion:
This study shows that adapting medical guidelines and strong media coverage played
a role in the clinical management of COVID-19 patients. At times, some ineffective
medications were prescribed such as hydroxychloroquine. Medical leaders and news coverage
should collaborate closely in future public health emergencies to prevent the prescription
of ultimately ineffective and potentially hazardous treatments.
Introduction
In 2019, a novel coronavirus first identified in Wuhan, China, brought the world to
a halt. Severe Acute Respiratory Syndrome (SARS) Coronavirus (CoV)-2 (SARS-COV-2)
continues to be a focus of worldwide news as the world slowly emerges from the pandemic
of 2020. The broad range of symptomatology of COVID-19, from mild upper respiratory
symptoms to severe acute respiratory syndrome and death, added uncertainty and fear
that fueled a desperate search for treatment. Clinicians in an overwhelmed healthcare
system were pressured to offer therapeutic options without clinical data. While the
race to vaccine development was underway, so were other therapies for both hospitalized
and clinic outpatients, as poor health outcomes and death was a common occurrence
at the pandemic's infancy. By April 2020, an estimated death toll of nearly 200,000
deaths was reported globally. In the interim of global research efforts, certain therapies
were administered without proven efficacy. In response to the increasing demand for
treatments, the Food and Drug Administration (FDA) issued Emergency Use Authorizations
(EUA) to allow the initiation of unproven therapies.
Initially, various treatments were explored in both inpatient and outpatient settings
based on anecdotal evidence or in vitro data. As clinical experience grew and results of clinical trials became available,
acceptance of evidence-based therapies varied and was influenced by clinicians' access
to clinical guidelines, popular perception, and patient expectations. As a result,
standard of care rapidly changed as coronavirus research elucidated the mechanisms
of the virus and the more effective treatment protocols developed over time. However,
there was a gap in research as there was no way to track these changes in a methodical
fashion, especially in the Texas healthcare system.
This project aimed to report how practice patterns changed over time in a cohort of
hospitalized patients in an academic center on the Gulf Coast of Texas. We examined
medication prescribing practices based on the type of clinical encounter: outpatient
(represented as pre-admission) compared with inpatient (during admission). Finally,
we discuss the media's role in influencing which therapies were used.
Methods
Study Population
Participants were recruited from University of Texas Medical Branch (UTMB) hospitals
in Galveston County from March 2020 to June 2021. Subjects consented to participate
in either the Observational Protocol for Diseases and Exposures of Public Health Importance
or the Clinical Characterization Protocol for Severe Emerging Infections and data
was maintained in the UTMB Biorepository for Severe Emerging Infections (BSEI). Eligibility
for participation in either protocol included confirmed or suspected infection with
a pathogen of interest and English speaking. Consent was provided either by the subject
or a legally authorized representative in writing. Only subjects with a confirmed
SARS-CoV-2 infection were included in this analysis. No subjects were consented for
enrollment for the study in April 2021; therefore, no data is available for this month.
Data Collection and Analysis
Data were collected by retrospective chart review and focused on patients admitted
for treatment of COVID-19 infection. Each chart was reviewed by at least two researchers
and compiled using REDCap. Medications reported by the patient as taken or prescribed
for the current illness before admission were recorded, as well as medications administered
during hospital admission based on chart review. Medications reported as outpatient
may have been prescribed or recommended by providers outside of the UTMB Health system.
Medications were categorized as: antiviral, antibiotic, steroid, supplement, antibody,
hydroxychloroquine (HCQ), or other. Antibiotics were only included if administered
within the first 48 hours of hospitalization as later treatment could indicate use
for a hospital-acquired infection.1, 2 UTMB participated in the Adaptive COVID-19 Treatment Trial (ACTT) which tested the
efficacy of remdesivir.3 Some study participants were enrolled in ACTT, and the use of remdesivir was counted
as intention-to-treat. Disease severity was categorized by the highest oxygen therapy
required: mild (no oxygen), moderate (nasal cannula), severe (high-flow nasal cannula,
CPAP, BiPAP), and critical (mechanical ventilation, ECMO). The percentage of participants
receiving each medication was calculated based on the month of admission to the hospital.
Microsoft Excel was used for all calculations.
Results
Characteristics of Study Population
The cohort for this study mimics the population of the hospital catchment area for
which patients presented, with a slightly higher proportion of non-Hispanic blacks
and a lower proportion of non-Hispanic whites being represented. The cohort was 42%
female, and consisted of 27% non-Hispanic blacks, 46% non-Hispanic whites, and 26%
Hispanic whites Table 1. The average age was 56 years with a range of 22–91 years, and the median duration
of hospital stay was 7 days (range of 2–56 days). Disease severity from mild to critical
were represented in this cohort and one patient was included who was later determined
to be in the convalescent stage of disease Table 1.
Table 1.
Characteristics of the Study Population.
| Characteristic |
Value |
| Age (years), mean (range) |
56 (22–91) |
| Total Population (n) |
90 |
| Female, n (%) |
38 (42) |
| Race/Ethnicity, n (%) |
|
| Non-Hispanic Black |
24 (27) |
| Non-Hispanic White |
41 (46) |
| Hispanic White |
23 (26) |
| Native Hawaiian/Pacific Islander |
1 (1) |
| Length of Hospitalization (days) |
|
| Median |
7 |
| Range |
2 – 56 |
| Severity, n (%) |
|
| Mild (no need for Oxygen) |
17 (19) |
| Moderate (NC) |
37 (41) |
| Severe (HFNC, CPAP, BiPAP) |
22 (24) |
| Critical (MV, ECMO) |
13 (14) |
| Convalescent |
1 (1) |
Legend: The severity of the disease was determined based on the highest oxygen needs during
hospitalization. O2 – Oxygen; NC – nasal cannula; HFNC – high flow nasal cannula; CPAP – continuous positive
airway pressure ventilation; BiPAP – bilevel positive airway pressure ventilation;
MV – mechanical ventilation; ECMO – extracorporeal membrane oxygenation.
Summary of Therapeutic Use in Different Patient Care Settings
There were differences in the therapeutics used to treat COVID-19 infections based
on the setting of treatment Figure 1A. All therapeutics examined could be given in either setting, except for remdesivir.
Seventy-one (79%) patients in this cohort received or were intended to receive the
antiviral remdesivir during their hospitalization. In contrast, the one patient that
received an antiviral in the outpatient setting received a neuraminidase inhibitor.
Antibiotic, steroid, and supplement use were given in both settings but at a greater
rate during hospitalization. Antibiotics were used to treat 41 (46%) participants
during hospitalization, compared to 27 (30%) receiving antibiotics pre-admission.
Forty-six (51%) of participants received steroids in the inpatient setting, while
18 (20%) received steroids pre-admission. remdesivir was given during hospitalization
to all patients categorized with critical disease, and most of those categorized as
having moderate or severe disease Figure 1B. Only a quarter of those categorized as having mild disease received remdesivir.
Those receiving steroids prior to hospital admission were typically categorized as
having moderate disease Figure 1C. Treatment guidelines were followed for those receiving steroids during hospitalization,
being given to a large majority of those categorized as having severe or critical
disease Figure 1C. Supplements such as vitamin C, vitamin D, and Zinc were given to 17 (19%) and 26
(29%) patients in the outpatient and inpatient setting, respectively. No patients
in this cohort received antibody therapy during hospitalization; only 3 received antibody
therapy in the outpatient setting. HCQ use was infrequent, 2 patients in an outpatient
setting and 7 during inpatient stay. Across all categories, patients in this cohort
were more likely to receive treatment when hospitalized than in an outpatient setting.
Figure 1 (A, B, C).
Percentage of Cohort Using Specified Therapeutics Pre-admission (Outpatient) versus
During Hospitalization (Inpatient)
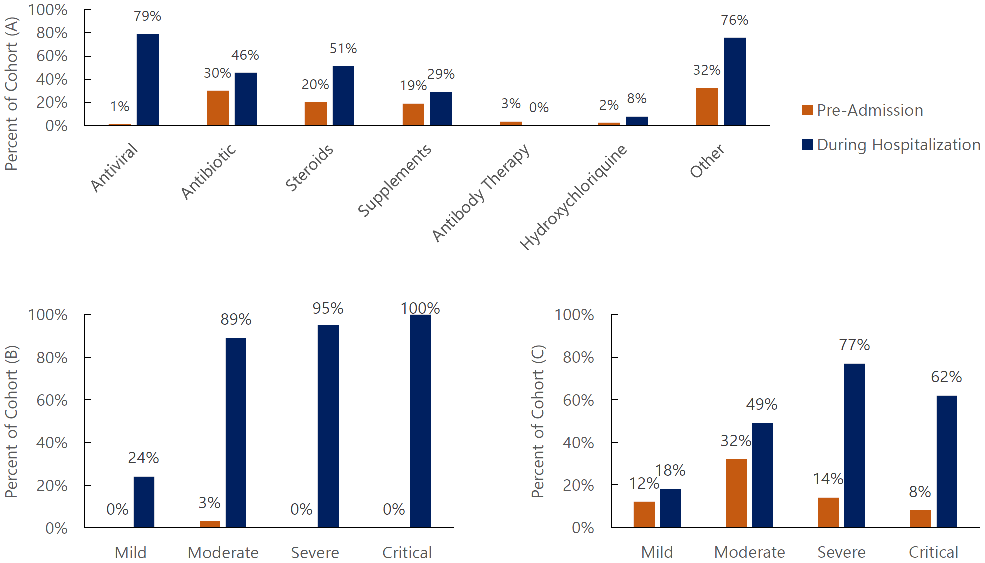
Legend: (A) Use of each therapeutic across entire cohort. (B) Use of antiviral therapy separated
by disease severity. (C) Use of steroid therapy separated by disease severity.
Therapeutic Use Over Time in the Outpatient Setting
In March 2020, antibiotics were favored in the outpatient setting Figure 2A. Antibiotic use declined over time and remained low throughout the rest of 2020.
Usage increased during January, February, and June 2021, with minimal use the rest
of 2021.
Figure 2 (A, B, C, D).
Percentage of Cohort Using Specified Therapeutics Before Admission (outpatient) Based
on Month of Admission.
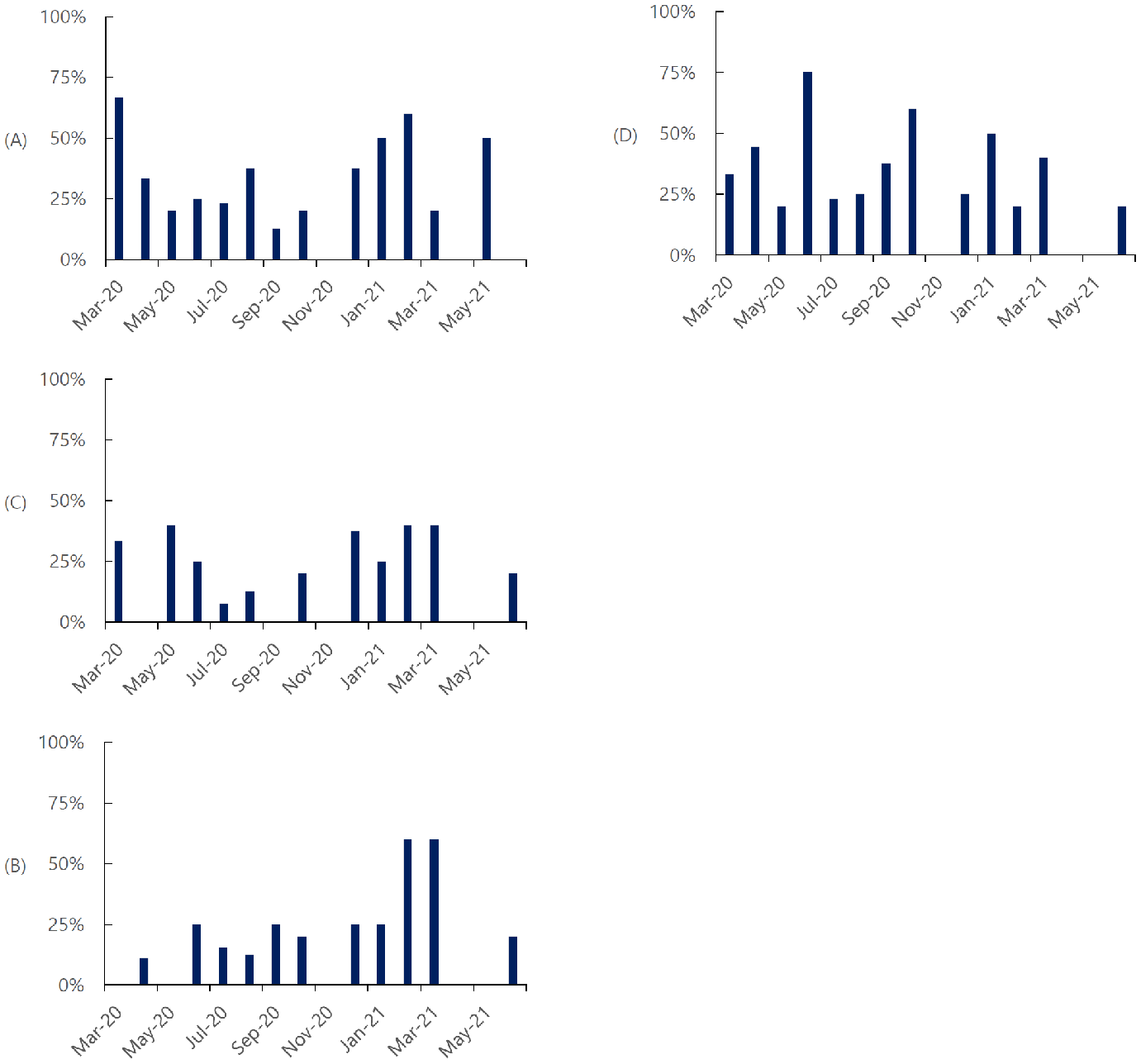
Legend: (A) Antibiotics, (B) Steroids, (C) Supplements, (D) Other (e.g., Pain Relievers,
Expectorants, Cough Suppressants, and Bronchodilators).
Initially, corticosteroid use for treating COVID-19 was debated due to previous concerns
after use to treat SARS in 2003. The NIH issued specific guidelines recommending against
the use of corticosteroids in non-hospitalized COVID-19 patients. Steroids were used
minimally in the outpatient setting from March 2020 to January 2021 Figure 2B. In February and March 2021, there was an increase to 60% of those hospitalized treated
with steroids. After this brief increase, few participants received steroids in the
outpatient setting. The trend of high steroid use does not correlate with the release
of the Randomized Evaluation of COVID-19 (RECOVERY) trial or NIH and IDSA.4 The results of the RECOVERY trial and inclusion of steroid use in NIH and IDSA guidelines
were limited to the inpatient setting. Steroid use was not recommended for outpatient
use. The increase in steroid use in early 2021 may have been due to a misunderstanding
of guidelines.
The use of supplements and other therapeutics (e.g., pain reliever, expectorant, cough
suppressant, bronchodilator) in the outpatient setting did not show a distinct trend
with respect to time Figure 2C-D. Many of these medications are available without a prescription, and it was not determined
if they were taken on the recommendation of a provider or at the patient's discretion.
Antibody therapy and HCQ were used infrequently in the outpatient setting. Twenty
percent of participants admitted in March 2021 and 40% in June 2021 received outpatient
antibody therapy; 13% in December 2020 and 20% in March 2021 received HCQ (data not
shown).
Therapeutic Use Over Time in the Inpatient Setting
Therapeutic use during hospitalization for COVID-19 followed a more predictable trend
than in the outpatient setting. UTMB circulated institutional recommendations for
treatment which typically followed NIH guidelines. remdesivir was given to most patients
after the FDA granted an EUA on May 1, 2020 Figure 3A. Before the issuance of the EUA, 83% and 44% of patients from this cohort in March
and April 2020, respectively, were counted as receiving remdesivir in an intention
to treat analysis as they were enrolled in ACTT-1.3 Even after the EUA was issued for Remdesivir, universal use of this medication was
not routine until December 2020.
Figure 3 (A, B, C).
Percentage of Cohort Using Specified Therapeutics During Hospitalization (Inpatient)
Based on Month of Admission

Legend: (A) Antivirals, (B) Steroids, (C) Antibiotics, (D) Other (e.g., Pain Relievers, Expectorants,
Cough Suppressants, and Bronchodilators).
Steroid use, specifically dexamethasone, in hospitalized patients was rarely utilized
until after the publication of the RECOVERY trial at the end of June 2020, showing
benefits in hospitalized patients receiving oxygen therapy Figure 3B.4 There was a clear increase in dexamethasone use in August 2020. Dexamethasone use
was given to most hospitalized participants for May 2021.
Antibiotic use in hospitalized patients fluctuated Figure 3C. Therapeutics categorized as “other” were used consistently from March to October
2020 Figure 3D. Except for January and June 2021, the use of these medications was lower in 2021
compared to 2020. HCQ use was low in the inpatient setting, with only 7 patients receiving
this therapy across the period analyzed (data not shown). Only one patient from this
cohort received HCQ in 2021. Also categorized as “other” were anticoagulants. Use
of anticoagulants, either prophylactically or therapeutically in hospitalized patients
was low in this cohort. Prophylactic use was more common in patients categorized as
having mild or moderate disease (10% and 13%, respectively) than those with severe
or critical disease (4% and 1%, respectively). Therapeutic doses were only given to
eight patients, one each for mild or moderate disease and three each for severe and
critical disease.
Discussion
In three years, robust research centered on COVID-19 quickly resulted in guidelines
based on clinical trial results for hospitalized and ambulatory patients. A retrospective
look of these efforts to find an effective treatment is worth the discourse. The FDA
issued several EUAs for the treatment of COVID-19 based on available data at the time,
indicating these therapeutics could provide some benefit to patients. The cohort examined
in this study offers insight into the therapeutics used to treat hospitalized COVID-19
patients at UTMB over the first 15 months of the pandemic. We reviewed several treatment
types, including therapies that received an EUA and those touted as beneficial in
mainstream media.
Our study found that antiviral, antibiotic, steroid, and supplement therapies saw
greater use in the inpatient setting from March 2020 to June 2021. Antiviral therapy
with remdesivir was more common in the inpatient setting since it was only available
in this setting at the time of the study. Treatment with remdesivir is now available
for non-hospitalized patients but requires access to a clinic capable of giving infusions
over multiple days. Our study noted a greater use of antibiotics in outpatient settings,
particularly azithromycin (AZM). Macrolides and HCQ were two frequently used antimicrobials
in countries such as France and China. This is a surprising reality given the lack
of data for their use in treating viral infections.5 Although macrolides are commonly used to target a variety of bacterial infections,
there is little reason to believe such a treatment would benefit a viral infection
such as COVID-19. Similarly, HCQ is commonly used to treat autoimmune disorders and
infections involving intracellular bacteria. One reason these therapeutics were considered
could have been due to strong media coverage when COVID-19 treatments were unknown.
The knowledge that certain micronutrients boost immunity likely influenced the use
of supplements categorized as ‘other’ in our study such as vitamin C and Zinc. However,
a third of patients reported having initiated these therapies before admission. Our
data did not ascertain whether these medications used before hospital admission were
initiated by a provider or at the patient's discretion.
When examining trends over time, clear patterns emerged in both settings. The data
support the idea that the presentation date may influence the extent of use of any
therapy. For example, in early 2020, when testing for COVID-19 was limited and community
spread was presumed low, antibiotics were prescribed more heavily in the outpatient
setting. Antibiotic use slowly declined as the year progressed but increased again
starting in December 2020. This increase coincides with the second major wave of cases
reported in the county from which this cohort resided. The use of steroids in the
outpatient setting was not prominent throughout the period examined. Still a brief
increase in use was seen during the first few months of 2021, when nearly 60% of patients
received outpatient steroid therapy. Finally, supplements had no discernable pattern,
with usage oscillating from month to month.
The EUA for remdesivir monotherapy, released on May 1, 2020, coincides with the greatest
use in this cohort of patients.6 In a large, randomized, placebo-controlled double-blinded trial, a statistically
significant decrease in recovery time was found for the group receiving remdesivir.3 The study thereby supported remdesivir's effectiveness in reducing recovery time
in hospitalized patients infected with SARS-CoV-2. This led to the issuance of an
EUA and eventual FDA approval with the addition of data from other trials. Another
notable benefit of remdesivir was seen in patients receiving low-flow oxygen therapy,
suggesting the antiviral prevented disease progression, as there was a lower frequency
of patients needing higher-level oxygen therapy and other respiratory support.3
In the open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial that
analyzed multiple therapies in hospitalized patients with COVID-19, the use of dexamethasone
was compared to usual care alone, with 28-day mortality as the primary outcome.4 The study found that significantly fewer patients died within 28 days in the dexamethasone
treatment arm than those receiving standard of care. Results from this trial led to
the recommendation of dexamethasone for all hospitalized adults requiring supplemental
oxygen.
Overall, the use of antivirals and steroids from July 2020 to March 2021 in this cohort
coincided with data releases for both remdesivir and dexamethasone and treatment guidelines,
such as those published by the Infectious Diseases Society of America (IDSA), the
National Institutes of Health (NIH), and the World Health Organization (WHO).7-9 In the outpatient setting, dexamethasone and other corticosteroids were widely used
as it was thought that their use would limit systemic inflammation.10 Increased use continued even after the results of the RECOVERY trial were released
and NIH guidelines were updated.
Another early consideration for COVID-19 treatment was the hydroxyl derivative of
chloroquine, HCQ, due to its potential immunologic benefits, such as in vitro inhibition of toll-like receptor signaling and alteration of cellular pH.11 An EUA was issued on March 28, 2020, for using HCQ to treat COVID-19;12 however, several studies revealed its limited clinical benefit. One retrospective
analysis of a large data set from over 96,000 patients found that HCQ did not offer
any therapeutic benefit and could reduce survival by potentiating ventricular arrythmia,
thus increasing the risk of invasive ventilation or death. Given these safety concerns,
the WHO removed HCQ as a treatment arm in the Solidarity trial. Its EUA was subsequently
revoked three months after its release.13
Although primarily a respiratory virus, COVID-19 often showed coagulopathy. It is
thought that during severe disease caused by the virus, dysregulated thrombosis cascade
within the alveoli and pulmonary vessels resulted in an initial local hypercoagulability
that then metastases.14 COVID-19 was also found to result in cellular abnormalities such as lymphopenia,
the degree of which correlated to disease severity.14 One theory proposed that the mismatch in neutrophil to lymphocytes ratio triggered
venous thrombosis and was a reliable predictor of mortality. Only a small percentage
of the cohort examined in this study was given anticoagulation medication, either
at prophylactic or therapeutic doses.
Monoclonal antibodies were not heavily used in this cohort, despite EUA. This is likely
because the EUA letters were released later in 2020 and early 2021. In this study,
bamlanivimab and etesevimab, which had EUAs released in February 2021, were used more
frequently pre-admission than during, which follows one of the use limitations proposed
by the FDA as neither had been studied in hospitalized patients.15
Among the treatments that had not received indications for use in COVID-19 infection,
antibiotics had a fair amount of use in our cohort that notably varied over time.
Benefits from AZM may be attributed to its mechanism of reducing the production of
intercellular adhesion molecule synthesis (e.g., ICAM-1), a component crucial for
viral adhesion.16 However, this effect has not been well studied for use in SARS-CoV-2 infection. The
studied effects of AZM came mainly from its adjunctive use with HCQ, as it was found
that both together showed some ability to interfere with viral replication, as evidenced
by a small clinical trial in France.16 Among a few randomized trials that investigated the therapeutic benefits of AZM as
monotherapy, one in 2021 conducted on 263 COVID-positive patients found that on day
14, no improvement or absence of symptoms was reported after a single dose of AZM.17
In our study, participants also had a considerable use of supplements. Several studies
support the benefit of Zinc for its anti-inflammatory properties, aiding in the production
of cytokines and improving the integrity of cellular tight junctions.18 Similarly, vitamin C is a notable antioxidant that influences immune cell migration
and function.19 Thus, the frequent use seen in outpatient and inpatient settings was well supported
in vitro.
Currently, several well-researched therapies are now accepted for COVID-19 therapy.
Among these are intravenous remdesivir for hospitalized patients and baricitinib,
an immune modulator. Although not heavily utilized in this cohort, convalescent plasma
(CP) appeared to be a promising COVID-19 therapeutic early in the pandemic. CP received
an EUA in August of 2020.20 Given its variable therapeutic efficacy, in February 2021, the EUA for CP was revised
to restrict its use only to hospitalized patients with poor humoral immunity and those
in the early stage of infection.21 Presently, the NIH advises against its use in immunocompetent hospitalized patients
and CP collected before the omicron variant surge and its use in immunocompetent hospitalized
patients.8, 22 In the outpatient setting, the NIH led the Clinical Trial of COVID-19 Convalescent
Plasma in Outpatients (C3PO) showed that CP offered little in the way of disease prevention
when given in early disease. In February 2021, the trial was discontinued as little
efficacy was found.23 The RECOVERY trial also showed minimal benefit of high-titer CP.24
Throughout 2020 to 2021, adjusting guidelines, media, and other factors increased
or decreased the use of certain therapeutics to treat COVID-19. Changes in the IDSA
guidelines show how therapeutic recommendations have been adjusted over time.7 As the pandemic progressed, additional research provided further guidance, contributing
to updated treatment guidelines and an improved standard of care.25 Furthermore, in the cohort examined here, there was a minimal delay in implementing
guidelines and the corresponding changes in clinical practice. For example, the IDSA
guidelines provided a strong recommendation for the use of dexamethasone on September
25, 2020. This change was evident in Figure 3B, with inpatient steroid use increasing from September to October 2022.
When looking retrospectively at the progression of COVID-19 therapeutics, one cannot
ignore the media's role, as unproven treatments were marketed through both mainstream
and online media. One example was seen on October 1, 2020, when former President Donald
Trump tested positive for COVID-19 and was treated with the antibody cocktail REGEN-COV
(Casirivimab and Imdevimab), remdesivir, and steroids, in addition to vitamin D and
Zinc.26 This could correlate to several spikes in medication usage seen in October 2020 and
the months immediately after, due to heavy media coverage. After Donald Trump was
treated with remdesivir monotherapy, inpatient antiviral usage increased and remained
high for this group Figure 3A. While this does not indicate causation, the positive media coverage potentially
increased patient willingness towards this treatment. Although not observed in this
cohort, the media may have influenced changes in the use of HCQ and AZM, especially
early in the pandemic. President Trump made statements regarding his use of HCQ and
AZM to prevent illness from COVID-19 and publicly pressured the FDA to release an
EUA for HCQ.27, 28 This sentiment was further publicized by the group America's Frontier Doctors, whose
own press conference went viral on social media. This struggle between media figures
and scientists led to the public questioning what was true, prompting some to demand
specific treatments when receiving care.
Overall medication use in the inpatient setting in this cohort mimicked what would
be expected based on changing clinical guidelines. The outpatient use of medications
showed a limited knowledge of disease etiology early in the pandemic, with antibiotics,
unproven supplements, and “other” therapeutics regularly being used. The outpatient
use of HCQ, AZM, Vitamin D, and Zinc for the treatment of Donald Trump showed a correlated
increase in October 2020. Similar increases were seen after other notable press events
and when NIH guidelines were adjusted to include or exclude certain medications, possibly
pointing to the effect that media coverage may have on medication use. In the face
of a new disease, it is important to provide treatments based on scientific and clinical
data rather than anecdotal evidence, and to communicate these findings with patients
to ensure safe and productive treatment.
Our study reveals that the evolution of COVID-19 treatment guidelines has been significantly
influenced by emerging clinical trial data, regional healthcare practices, and the
varying interpretations of these data by medical experts. This observation aligns
with previous findings which also note rapid guideline updates in response to major
clinical trial outcomes.29 For instance, the swift incorporation of findings from the RECOVERY trial into treatment
guidelines reflects a broader trend of integrating high-quality evidence into clinical
practice. Moreover, our analysis highlights regional variations in the adoption and
implementation of treatment guidelines, which corroborates the work of Lee et al.30 They emphasized the challenges of aligning global recommendations with local healthcare
infrastructures and patient demographics. These variations underscore the necessity
for flexible and adaptive guideline frameworks that can accommodate regional differences
while maintaining a foundation in robust scientific evidence.
Limitations
Our study was limited to patients hospitalized at UTMB in Galveston County and may
not represent the larger population. Further studies could benefit from a multi-center
approach to encompass a broader demographic and geographic pool and add generalizability
to the study. Additionally, patients who chose to participate in this research study
may have been more open to receiving other therapeutic interventions, including medications
undergoing clinical trials, even if they were not authorized or proven, potentially
resulting in bias. Lastly, the data were collected retrospectively, and may be subject
to recall bias. These limitations could be addressed through further patient outreach
and using a broader cohort in future research.
Summary – Accelerating Translation
In this article titled “A Descriptive Analysis of the Use of Various Therapeutics
in a Cohort of COVID-19 Patients,” the authors investigated how a novel disease such
as COVID-19 was clinically treated when national guidelines constantly changed. By
understanding how hospital systems such as UTMB treat novel illnesses with mixed guidelines,
future new diseases can be more effectively and efficiently managed. Being adaptable
and implementing guidelines is an important aspect of medicine, as new diseases will
likely emerge. The authors followed 90 patients with a positive COVID-19 diagnosis
from March 2020 to June 2021. They collected a detailed accounting of what medications
each patient took before admission, after admission, and if the patient was hospitalized.
This data showed large differences were seen in patients who were managed in outpatient
clinics versus in the hospital. Antibiotics such as AZM were given much more commonly
in the outpatient setting despite a lack of guidelines for administering antibiotic
treatment for COVID-19. When analyzing the data month to month, it was clear that
guidelines and news coverage played a significant role in how physicians treated COVID-19
through 2020. Medications that received strong media coverage such as hydroxychloroquine,
were prescribed noticeably in the months shortly after news coverage. This is despite
no recommendation from national and local guidelines at the time, which would be later
updated to recommend against the use of hydroxychloroquine. This descriptive analysis
encourages policymakers in the United States to work closely with physicians when
communicating the best treatment recommendations for a novel disease. A unified message
to the medical community, media, and public would strengthen strong clinical treatment
practices and prevent the use of ineffective medications.
Acknowledgments
We would like to thank every individual who has donated to the UTMB Biorepository
for Emerging Infectious Diseases. Your contributions are advancing science and medicine
to help our future patients.
Conflict of Interest Statement & Funding
Funding for this study came from departmental funds from the University of Texas Medical
Branch, Department of Internal Medicine – Division of Infectious Diseases.
Author Contributions
Conceptualization: AM, BM, SM, and CL. Investigation: AM, BM, JC, DM, RD, TG, SO,
and CL. Supervision: SM and CL. Writing – Original Draft: AM, BM, and CL. Writing
– Review & Editing: JC, DM, RD, TG, SO, and SM.
References
1. Monegro AF, Muppidi V, Regunath H. Hospital-Acquired Infections. StatPearls. Treasure Island (FL) 2023.
2. Revelas A. Healthcare - associated infections: A public health problem. Niger Med J. 2012;53(2):59–64.
3. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813–26.
4. Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
5. Recovery Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383(21):2030–40.
6. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment [press
release]. 05/01/2020 2020.
7. Infectious Diseases Society of America. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Last updated Aug 12, 2024; cited May 27, 2021.
8. National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available from: https://www.ncbi.nlm.nih.gov/books/NBK570371/. Last updated Mar 1, 2024; cited Apr 21, 2021.
9. World Health Organization. Therapeutics and COVID-19: Living guideline. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.2. Last updated Nov. 10, 2023; cited Sept. 2, 2020.
10. Bradley MC, Perez-Vilar S, Chillarige Y, Dong D, Martinez AI, Weckstein AR, et al Systemic Corticosteroid Use for COVID-19 in US Outpatient Settings From April 2020
to August 2021. JAMA. 2022;327(20):2015–8.
11. Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6
monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6):105982.
12. Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020 [press release]. 03/30/2020 2020.
13. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine
[press release]. 06/15/2020 2020.
14. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res. 2020;194:101–15.
15. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19 [press release]. 02/09/2021 2021.
16. Parra-Lara LG, Martinez-Arboleda JJ, Rosso F. Azithromycin and SARS-CoV-2 infection: Where we are now and where we are going. J Glob Antimicrob Resist. 2020;22:680–4.
17. Oldenburg CE, Pinsky BA, Brogdon J, Chen C, Ruder K, Zhong L, et al Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2
Infection: A Randomized Clinical Trial. JAMA. 2021;326(6):490–8.
18. Diyya ASM, Thomas NV. Multiple Micronutrient Supplementation: As a Supportive Therapy in the Treatment of
COVID-19. Biomed Res Int. 2022;2022:3323825.
19. Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, et al Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020;12(12).
20. FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID-19 Treatment,
Another Achievement in Administration's Fight Against Pandemic [press release]. 08/23/2020 2020.
21. FDA In Brief: FDA Updates Emergency Use Authorization for COVID-19 Convalescent Plasma to Reflect
New Data [press release]. 02/04/2021 2021.
22. Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, Tzanninis IG, et al Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2021;21(2):167–79.
23. Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al Early Convalescent Plasma for High-Risk Outpatients with Covid-19. N Engl J Med. 2021;385(21):1951–60.
24. Recovery Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised
controlled, open-label, platform trial. Lancet. 2021;397(10289):2049–59.
25. Potter GE, Bonnett T, Rubenstein K, Lindholm DA, Rapaka RR, Doernberg SB, et al Temporal Improvements in COVID-19 Outcomes for Hospitalized Adults: A Post Hoc Observational
Study of Remdesivir Group Participants in the Adaptive COVID-19 Treatment Trial. Ann Intern Med. 2022;175(12):1716–27.
26. Cohen J. Update: Here'whats what is known about Trump's COVID-19 treatment. 202010/05/2020.
27. abcnews. Timeline: Tracking Trump alongside scientific developments on hydroxychloroquine. Available from: https://abcnews.go.com/Health/timeline-tracking-trump-alongside-scientific-developments-hydroxychloroquine/story?id=72170553 Last updated Aug 8,2020; cited Aug 8, 2020.
28. The Guardian. Trump is taking hydroxychloroquine, White House confirms. Available from: https://www.theguardian.com/us-news/2020/may/19/trump-hydroxychloroquine-covid-19-white-house. Last updated May 19, 2020; cited May 19, 2020.
29. Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023;22(6):449–75.
30. Cokljat M, Cruz CV, Carrara VI, Puttaraksa K, Capriglioni C, Insaurralde SM, et al Comparison of WHO versus national COVID-19 therapeutic guidelines across the world:
not exactly a perfect match. BMJ Glob Health. 2024;9(4).
Alfred A. Mathew, 1 MD, MBA. University of Texas Medical Branch, Galveston, TX, USA.
Barbara Mensah, 2 MD, University of Texas Medical Branch, Galveston, TX, USA.
John C. Cravero, 2 MD, University of Texas Medical Branch, Galveston, TX, USA.
David C. Moffatt, 2 MD, University of Texas Medical Branch, Galveston, TX, USA.
Roshan Dongre, 3 BS, Texas A&M University School of Engineering Medicine, Houston, TX, USA.
Thao K. Giang, 2 MD, University of Texas Medical Branch, Galveston, TX, USA.
Samantha C. Olvera, 2 MD, University of Texas Medical Branch, Galveston, TX, USA.
Susan L.F. McLellan, 4 MD, MPH. University of Texas Medical Branch, Galveston, TX, USA.
Corri B. Levine, 5 PhD, MS, MPH. University of Texas Medical Branch, Galveston, TX, USA.
About the Author: Alfred Mathew is a recent medical graduate from the University of Texas Medical Branch
John Sealy School of Medicine, Galveston, USA. He is also a Certified Healthcare Financial
Professional accredited through the dual MD/MBA track. Barbara Mensah is a categorical
Medicine intern at Dell Medical School in Austin, currently undecided on a career
path. Notable recent awards/nominations include a nomination for Resident Educator
of the term.
Correspondence: Corri B. Levine. Address: 301 University Blvd, Galveston, TX 77555-0435, USA. Email:
cblevine@utmb.edu
*These authors contributed equally to this work and are listed as co-first authors.
Editor: Francisco J. Bonilla-Escobar;
Student Editors: Richard Christian Suteja, Cesare Mercall & Praise Senyuy Wah;
Proofreader: Amy Phelan;
Layout Editor: Julian A. Zapata-Rios;
Process: Peer-reviewed
Cite as
Mathew AA, Mensah B, Cravero JC, Moffatt DC, Dongre R, Giang TK, et al. A Descriptive
Analysis of the Use of Various Therapeutics in a Cohort of COVID-19 Patients and the
Influence of Media Coverage. Int J Med Stud. 2024 Jul-Sep;12(3):259-266.
Copyright © 2024 Alfred A. Mathew, Barbara Mensah, John C. Cravero, David C. Moffatt,
Roshan Dongre, Thao K. Giang, Samantha C. Olvera, Susan L.F. McLellan, Corri B. Levine
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 12, NUMBER 3, September 2024


