Review
A Narrative Review on the FSTL-1 Protein and its Current Known Impact on Cardiovascular
Ischaemic Disease
José Rodrigues Gomes1
doi: http://dx.doi.org/ijms.2024.2297
Volume 12, Number 4: 457-464
Received 19 09 2023;
Rev-request 06 12 2023;
Rev-request 08 07 2024;
Rev-recd 05 03 2024;
Rev-recd 29 08 2024;
Accepted 25 10 2024
ABSTRACT
This narrative review investigates the potential therapeutic role of FSTL-1 in addressing
severe cardiac issues following myocardial infarctions (MI). Despite advances in modern
medicine, MI persist as a leading global cause of death, with stem cell therapy falling
short of expectations since the early 2000s. In contrast, FSTL-1, an emerging bone
morphogenetic protein, demonstrates promise based on successful studies. We conducted
a qualitative narrative synthesis of studies published in PubMed, Scopus, and Web
of Science between January 2000 and May 2022. This research explores the intricate
scientific aspects of FSTL-1's contribution to myocardial regeneration, utilizing
a chronological approach to trace its progression from biological pathways to broader
scenarios. It examines the mechanisms regulated by FSTL-1 and its effects on cardiac
tissue and cells, highlighting its potential as a therapeutic agent emphasizing its
multifaceted role in cardiac regeneration. By deepening our comprehension of FSTL-1,
this study significantly contributes to knowledge advancement, offering insights into
its role in addressing severe cardiac issues post-MI. By consolidating current knowledge
and proposing new avenues for investigation, this work offers valuable insights into
FSTL-1's significance in advancing cardiovascular health and post-MI recovery.
Introduction
In 54 countries belonging to the European Cardiology Society, there were 19.9 million
new cases of cardiovascular disease (CVD) and 108.6 million individuals were found
to be suffering from CVD in 2017. Ischaemic heart disease (IHD) is identified as the
foremost expression of CVD, with 3.6 million new cases and 34.9 million individuals
living with IHD.1 CVD remains acknowledged as the leading cause of mortality in Europe, accounting
for 4.1 million deaths annually, representing 47% of all deaths among women and 39%
among men.1
Cardiac regenerative therapies have made significant progress over recent decades,
aiming to address the limitations of traditional treatments for myocardial infarction
(MI) and heart failure. Stem cell therapy has been a central focus, with various types,
including embryonic, induced pluripotent, and mesenchymal stem cells, explored for
their potential to replace damaged heart cells. However, challenges such as low engraftment
rates, limited functional integration, and the risk of tumorigenicity have hindered
widespread clinical success.2 Cell-free therapies, such as the use of exosomes, microRNAs, and growth factors,
have emerged as alternatives, targeting cardiac repair through cell signalling pathways.
Tissue engineering approaches, including cardiac patches and 3D bioprinting, aim to
create supportive environments for myocardial regeneration.3 Gene therapy, utilizing techniques like viral vectors and CRISPR/Cas9, offers another
avenue for promoting heart tissue repair by targeting specific pathways.4 Biomaterials, such as injectable hydrogels and scaffolds, provide structural support
and enhance cell survival, though challenges like immune response, vascularization,
and functional integration persist.5
Despite these advancements, the field faces ongoing hurdles that require further research
and innovation. Managing immune responses, ensuring adequate blood supply to regenerated
tissues, and achieving long-term efficacy and safety in humans are critical areas
of focus. Emerging approaches, including the investigation of novel biomolecules like
FSTL-1 and immunomodulation strategies, offer promising new directions. The ultimate
goal is to develop therapies that can reliably regenerate heart tissue, restore function,
and improve outcomes for patients with heart disease. While significant strides have
been made, translating these innovations into widely available clinical treatments
remains a complex and ongoing challenge.
This dual focus on both cell-based cardiac regeneration techniques and the potential
role of FSTL-1 underscores the urgency and significance of ongoing research endeavors
in addressing the formidable burden of cardiovascular disease, particularly ischemic
heart disease, in contemporary healthcare landscapes. The proposed objective of this
narrative review is to comprehensively explore the role of FSTL-1 in cardiac regeneration,
particularly within the context of ischemic heart disease. The review aims to synthesize
current findings on FSTL-1's mechanisms of action, its therapeutic potential in promoting
cardiac repair, and the challenges and opportunities for translating these insights
into clinical practice. By integrating insights from basic research, preclinical studies,
and emerging clinical evidence, the review seeks to provide a nuanced understanding
of how FSTL-1 could be harnessed to improve outcomes for patients with ischemic heart
disease.
Ischaemic Heart Disease
As mentioned before, Ischaemic Heart Disease (IHD) has been shown to be a severe pathology
with a high mortality rate. Within a simplistic approach, IHD is caused by the acute
occlusion of one or multiple sizable epicardial coronary arteries for more than 20
minutes, which can lead to an acute MI.7 Typically, necrosis spreads from the sub-endocardium to the sub-epicardium region.
Depending on the territory affected by the infarction, cardiac function is typically
compromised, and current treatment options are limited. Due to the minor renewal capacity
of the myocardium,8 the infarcted area heals by scar formation, and often, the heart is remodeled, characterized
by dilation, segmental hypertrophy of remaining viable tissue, and cardiac dysfunction.
The initial effects of oxygen deprivation will disrupt the sarcolemma arrangement
in heart muscle tissue and relaxation of myofibrils, shortly followed by alterations
in mitochondrial ultrastructure. These changes will then lead to mitochondrial dysregulation,
affecting energy availability. More advanced stages of prolonged ischaemia will result
in liquefactive necrosis of heart tissue, especially in the myocardium. The deposition
of collagen type I and type III in fibrosis is essential in the short term to stop
the rupture of ventricular walls; however, this mechanism makes it increasingly difficult
for the injured to maintain its functional capacity. This is due to its ill effects
on the ventricle's geometry, resulting in an accentuated loss of contractile and pump
function.9 Alongside this, an inflammatory reaction will also occur by motivating the migration
of macrophages to the myocardium,10 mainly through macrophage 1 and 2. Macrophage activity will be rich in several growth
factors and cytokines.10,11
Methods
In this narrative review, an analytical framework was adopted to synthesize and evaluate
the findings from the literature, aiming to depict the chain of logic, as evidence
must support possible future clinical outcomes. To refine the investigation, a comprehensive
search strategy was employed to identify relevant literature sources, utilizing specific
Medical Subject Headings (MeSH) terms provided by the National Library of Medicine
(NLM). These terms included Myocardial Ischemia, Coronary Artery Disease, FSTL-1,
Follistatin-Related Proteins, and Follistatin-Related Protein 1. The primary databases
utilized for the literature search included PubMed, Scopus, and Web of Science, with
a focus on articles published between January 2000 and May 2022 to capture the most
recent advancements in the field.
The inclusion criteria for this narrative review were rigorously defined to ensure
a comprehensive and relevant synthesis of existing literature on FSTL-1 in cardiac
regeneration. Eligible studies included peer-reviewed observational and interventional
studies, reviews, meta-analyses, and both molecular and animal model studies, all
published in English between January 2000 and May 2022. The focus on studies published
within this timeframe was chosen to capture the most relevant and contemporary research,
reflecting the significant advancements in the understanding of FSTL-1 over the past
two decades. This period was selected because it encompasses the critical years during
which FSTL-1 emerged as a potential therapeutic target, as well as the development
of advanced molecular techniques and animal models that have provided deeper insights
into its biological functions. By concentrating on this timeframe, the review aims
to highlight the progression of scientific knowledge and the most current perspectives
on FSTL-1's role in cardiac regeneration, ensuring that the findings are both up-to-date
and aligned with the latest research trends.
Selection was based on the studies' contributions to elucidating FSTL-1's mechanisms
within human physiology, with particular emphasis on research that provided clear
and significant insights into its role in cardiovascular regeneration. To ensure that
the included studies were of high relevance and scientific rigor, only those that
adhered to established principles of biomedical research were considered. This included
studies employing robust methodologies, validated models, and those that used precise
and accepted scientific terminology, ensuring the findings could be interpreted accurately
by medical students and professionals alike.
Additionally, studies were omitted if their methodologies were found to be flawed
or if their findings did not align with the broader body of evidence on FSTL-1. Methodological
flaws that led to exclusion included issues such as small sample sizes, inadequate
controls, lack of reproducibility, and insufficient statistical power, all of which
could compromise the validity and reliability of the findings. Studies with discrepancies
or inconsistencies when compared to the established scientific consensus on FSTL-1
were also excluded. This approach was taken to ensure that the review presents a cohesive
and scientifically sound narrative, grounded in evidence that is both credible and
widely accepted by the research community. By filtering out studies with methodological
weaknesses or conflicting results, the review aims to provide a clear and accurate
synthesis of the current state of knowledge on FSTL-1, thereby offering reliable insights
into its potential therapeutic applications.
The synthesis of findings followed a structured narrative approach, enabling a qualitative
analysis that integrated diverse types of evidence to construct a cohesive overview
of FSTL-1's role in cardiovascular regeneration. The narrative was developed chronologically,
reflecting the evolution of scientific knowledge from fundamental concepts to advanced
animal models and potential clinical applications, thereby providing a clear and logical
progression of the field's development.
Results
FSTL-1 in the Heart
The most prominent regulation mechanism by FSTL-1 is the serine/threonine protein
kinase (AKT), also known as phosphatidylinositol 3-kinase (PI3K). AKT has been identified
as a key piece in myocardial growth induced by stress (Fig.1).12,13 Investigation of AMP-activated protein kinase (AMPK) has also been conducted and
discovered to safeguard cardiomyocytes from apoptosis during a MI.14,15 This is due to the capacity of FSTL-1 to stimulate the phosphorylation of AMPK Thr172.14 Research has proven that FSTL-1 overexpression would lead to an upregulation of both
an AKT and ERK signalling in cardiac myocytes, resulting in better survival rates
under hypoxic conditions and induced apoptosis.16 This correlates with previous research, as AKT and ERK are positively correlated
with cellular survival.17,18,19 Other pro-survival factors include Pim-1, hypoxia-inducible factor-1α, and heme-oxygenase-1,
which are also involved in the AKT signalling mechanism, although their relationship
and function are still not understood in their entirety.20,21
Figure 1
Visual Representation of the Discussed FSTL-1 Stimulated Pathways.
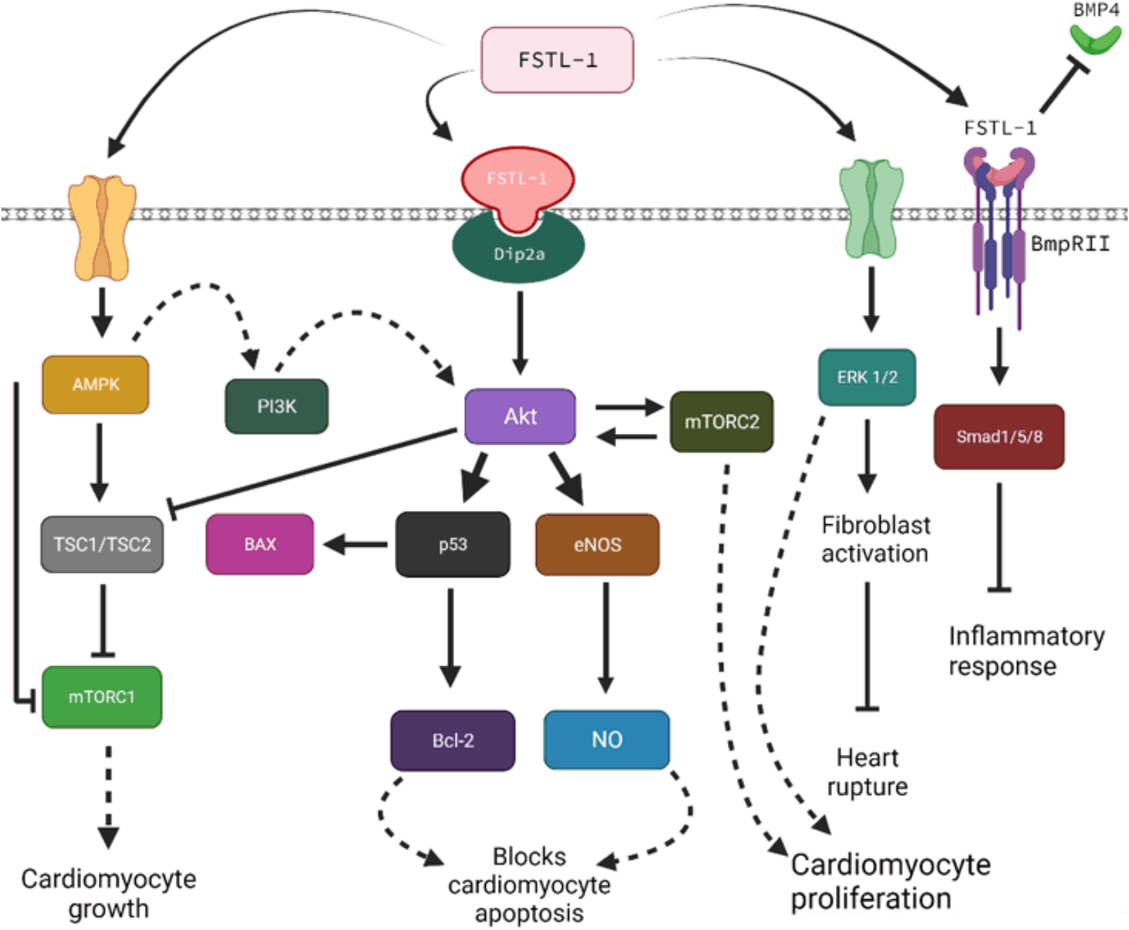
Legend: AMPK, AMP-Activated Protein Kinase; PI3K, Phosphoinositide 3-Kinase; TSC1/2, Tuberous
Sclerosis Complex 1/2; mTORC1/C2, Mammalian Target of Rapamycin Complex1/2; DIP2A,
Disco-Interacting Protein 2 Homolog A; Akt, Protein Kinase B; BAX, Bcl-2 Associated
X-protein; eNOS, Endothelial Nitric Oxide Synthase; NO, Nitric Oxide; ERK, Extracellular
Signal-Regulated Kinase; BMP-4, Bone Morphogenetic Protein-4.
Meanwhile, ERK signalling seems to occur mainly in cardiac fibroblasts, with its central
purpose being the proliferation and migration of the same cells. It is hypothesized
that the controlled fibrotic reaction offered by FSTL-1 derives from an early activation
and migration of cardiac fibroblasts, which in turn will lead to a greater myofibroblast
build-up in the infarcted area.22 This is believed to allow for an improved synthesis and maturing of extracellular
matrix in the affected zone.14 This reasoning is well-based, as FSTL-1 largely resembles the SPARC family, which
is an initial controller of extracellular matrix maturation after MI.23 Although alternative pathways to that proposed by Murayama et al. might also exist
in relation to the activation of FSTL-1. As it has been demonstrated that fibroblasts
are responsible for activating the Smad2/3 signalling route via TGF-β1 which will
cause a fibrotic response.22
Bone morphogenic protein-4 (BMP4) has also been shown to boost the apoptosis of cardiomyocytes.23 BMP4 is one of the commonly released proteins during an inflammatory response to
MI24,25 and is related to an enhanced phosphorylation of the previously mentioned Smad1/5/8
signalling, as shown in Figure 1. Reports have been conducted where they showed that FSTL-1 would bind to BPM426, which would inhibit further activity.14,27
Inflammatory Response
Macrophages are the main source of proinflammatory cytokines during MI.28,29 Cytokines such as IFNγ and IL-1β will increase the secretion of FSTL-1. Other cytokines
are responsible for increased levels of tumour necrosis factor-α (TNF-α) and interleukin-6
(IL-6). BMP4 is largely known for its increase in the expression of TNF-α and IL-6
after MI, resulting in an exaggerated and prejudicial inflammation of cardiac tissue.
Due to the inhibition of BMP4 by FSTL-1 (Fig.1), inflammatory processes are considerably decreased, thus proving that FSTL-1 is
a strong anti-inflammatory in post-MI.14 AMPK signalling, dependent on FSTL-1, has also been linked to an inhibition of macrophage
migration (Fig.1).15 FSTL-1 also decreased lipopolysaccharide-stimulated expression of proinflammatory
genes via activation of AMPK.14
Real-World Response
In a forerunner investigation by Wei et al., FSTL-1 was introduced to the epicardium
through a nanofibrillar collagen patch.34 It proved some different short-term effects, including reduced fibrosis and increased
vascularisation beneath and surrounding the epicardial patch. Measurements showed
an increase in the number and size of blood vessels. It is believed one of the processes
behind revascularisation depends on a nitric-oxide process, which is most likely regulated
by a paracrine mechanism.30 Cardioprotection also occurred, as embryonic stem cell-derived cardiomyocytes did
not undergo apoptosis provoked by the hypoxic environment, which is in correlation
with previous studies.16,30
Wei et al. also proved that it could have mid-term effects, as after a 4-week period,
the area beneath the patch showed striated myocytes, and in the border zones of the
patch, cardiomyocytes had also undergone cell division, which proved that FSTL-1 had
successfully induced cell cycle entry and cytokine release.33 However, Chen et al. also showed that FSTL-1 can be used to incentivise the proliferation
of mature adult ventricular cardiomyocytes, as it did not induce synthesisation of
DNA or division as well as hypertrophy, showing some limitations in this aspect.34 Thus, clear knowledge gaps still remain, as currently it is still unknown if these
cells originate de novo or from pre-existing cardiomyocytes. Studies have been conducted in this regard,
as fate mapping indicated that resident cardiomyocytes were the main source of regeneration
in the myocardium. However, during the investigation, a small percentage of cardiomyocytes
didn't undergo labelling, suggesting an alternative source of cardiomyocytes.35 Current evidence points that this unknown source is most likely made up of ckit+
cells, as it was found that they can also contribute to the proliferation and regeneration
of cardiomyocytes after MI.36
Probably the most pertinent issue that came from Wei et al. was the difference between
FSTL-1 expression in the myocardium vs. the epicardium. The overexpression of myocardial FSTL-1 varied in its role in comparison
to the overexpression of FSTL-1 in the epicardium. The initial idea behind this discrepancy
was that there were differences in the migration rates of the cells due to differing
glycosylation processes. FSTL-1 produced from different cellular sources are most
likely exposed to differences in the post-translational glycosylation, which will
inevitably result in varying isoforms.37 FSTL-1 derived from the myocardium demonstrated cardioprotective functions but not
cardio regenerative.38 While FSTL-1 derived from the epicardium demonstrated a cardio regenerative capacity.38 Other studies showed that non-glycosylated FSTL-1 increases the proliferation of
cardiomyocytes, while glycosylated FSTL-1 protects cardiomyocytes from peroxidase-induced
apoptosis.38 However, other factors might also be influencing the activation processes and subsequent
role of FSTL-1. Maruyama et al. in a more recent investigation, explored this topic
as they evaluated the effect of glycosylation of FSTL-1 in relation to cardiac fibroblast
activation.39 For this, they used insect, mammalian, and bacteria cells. Although the glycosylation
mechanisms varied substantially between the three, there were no statistically significant
differences in the capacity of each FSTL-1 protein to promote activation of cardiac
fibroblasts and their role.39
Other areas of interest revolve around the relationship of FSTL-1 with other peptides,
such as thymosin β4, due to their similarities, such as the production of epicardial-derived
cells and a strong driving force of angiogenesis and mobilization.38 Thymosin β4 has already been reported as a strong pro-vasculogenic factor.40 Following MI, thymosin β4 has been shown to induce epicardial-derived cells to form
vascular precursors and prompt angiogenesis in the human heart.41 Further investigation has established a relationship between thymosin β4 and the
capacity of Wt1+ cells to undertake cardiomyogenesis.40,42
Alongside this, FSTL-1 has been proven to inhibit the entrance into the apoptotic
mode of cardiomyocytes. Akt/GSK-3β signalling was verified in hypoxic-FSTL-1 cells,
being currently held as the main mechanism behind anti-apoptosis in hypoxic conditions.
More technical analyses have also shown that the heart upregulated FSTL-1 expression
under mechanical stresses such as pressure.34 Due to these cardioprotective roles, cardiac tissue, under the presence of FSTL-1,
can be able to positively regulate the cardiac microenvironment and induce the importation
or production of pro-regenerative substances.34 This regulation post-MI is managed by elements such as retinoic acid, hypoxia-inducing
factor-1α, fibroblast growth factors, transforming growth factor-beta (TGFβ), insulin-like
growth factor, and BMP, among many others.43
Musculoskeletal Secretion
FSTL-1 produced outside the heart is also essential in post-MI scenarios, as skeletal
muscle is the main producer of circulating FSTL-1 and has been linked to transgenic
AKT-1 overexpression.44 Circulating FSTL-1 will resemble a myokine by maintaining its previously reported
cardioprotective role through an endocrine method.45 Increased myocardium angiogenesis and an overall increase in the heart's performance
were reported due to an augmented TGFβ-Smad2/3 signalling (Fig.1).45,46 Reduced levels of FSTL-1 in the blood have also been shown to be correlated to more
prominent inflammatory reactions in arterial injury.46 FSTL-1 will also inhibit the proliferation and migration of smooth muscle cells in
damaged blood vessels, which will lower the effect of neointimal hyperplasia after
vascular injury.45 This will result in the control of smooth muscle cells through the stimulation of
AMPK mechanisms through an increase in phosphorylation.45 Exercise is essential as it increases FSTL-1 expression in skeletal muscle, especially
through intermittent aerobic exercise.46 This is further emphasised as circulating FSTL-1 would increase its expression in
the heart's myocardium heart, which is a strong indicator of regulation through positive
feedback.45 FSTL-1 has also been tested in conjunction with mesenchymal stem cells due to the
inherent characteristics FSTL-1 possess.30 This was an attempt at addressing some of the issues currently associated with cardiac
stem cell therapy, mainly low survival percentage and difficulty in retention and
engraftment of donor cells in ischemic tissue.7 A study by Shen et al. showed promise in this hypothesis, as FSTL-1 successfully
enhanced the resistance of mesenchymal stem cells to hypoxia, a reduction in fibrosis,
inflammatory cell infiltration and an increase in neovascularisation.30 The production of extracellular matrix was also considerably modulated, as tested
by western blot analysis, which showed that mesenchymal stem cells injected in conjunction
with FSTL-1 decreased the transcription rates of collagen type I and fibronectin in
peri-infarcted zones. Other pro-fibrogenesis cytokines, such as connective tissue
growth factor, decreased in ischaemic myocardium.30
Discussion
In evaluating the limitations of the studies encompassed within this review, several
critical considerations emerge that significantly influence the interpretation of
findings and the broader understanding of FSTL-1's role in cardiac regeneration. A
primary concern is the diverse methodologies and experimental models employed across
these studies. Animal models, such as rodents and larger mammals, have been invaluable
in elucidating the molecular mechanisms underpinning cardiac regeneration. However,
the translational relevance of these findings to human physiology remains a complex
challenge. Differences in cardiac anatomy, physiology, and regenerative capacity between
species necessitate cautious extrapolation of preclinical data. Many investigations
have provided valuable insights into the molecular pathways involving FSTL-1 in cardiac
regeneration, a predominant focus on mechanistic aspects without sufficient consideration
of direct clinical relevance or translational implications limits the practical utility
of these findings. Additionally, the heterogeneity in experimental protocols—ranging
from dosing regimens and delivery methods to the timing of interventions—introduces
variability that complicates the interpretation and comparison of results across studies.
This emphasis on fundamental research, though crucial for understanding underlying
biological mechanisms, can often fail to address the practical challenges of translating
FSTL-1-based interventions into clinically effective therapies. The absence of comprehensive
preclinical studies that integrate mechanistic insights with translational endpoints—such
as functional recovery and long-term safety assessments—represents a significant gap
in the literature.
Furthermore, publication bias poses a significant challenge in the existing literature.
Studies reporting positive outcomes or novel findings are more likely to be published,
potentially leading to an overrepresentation of favorable results. Conversely, studies
with neutral or negative findings may face greater difficulties in publication, resulting
in an underrepresentation of valuable data. This bias can skew the overall perception
of FSTL-1's therapeutic potential, either fostering unwarranted enthusiasm or undue
skepticism.
In addition to the aforementioned points, the integration of FSTL-1 into complementary
therapeutic approaches, such as mesenchymal stem cell therapy, holds promise for optimizing
cardiac regeneration strategies. Leveraging the epicardium as a targeted cell therapy
site represents a compelling avenue for enhancing the efficacy of regenerative interventions.
However, to fully capitalize on this potential synergy, a comprehensive understanding
of the intricate molecular networks governing cardiac regeneration is essential. These
networks, characterized by intricate interplay and feedback loops, involve not only
FSTL-1 but also other cardio-protective and regenerative proteins expressed in the
myocardium. Despite significant progress in elucidating the mechanisms regulated by
FSTL-1, gaps persist, particularly regarding the upstream activators of FSTL-1 expression.
Resolving these knowledge gaps is critical for developing precise therapeutic interventions.
Furthermore, while recent studies have shed light on FSTL-1's role in modulating immune
responses post-MI, further research in this direction is warranted to unravel its
full therapeutic potential.
Moreover, fostering interdisciplinary collaborations and aligning research efforts
with emerging trends in cardiovascular science is paramount. Recent advancements in
stem cell therapy, including investigations into c-kit+ cells and Wt1+ cells, present
opportunities to address existing challenges, such as poor engraftment of donor cells
in hypoxic environments. Exploring the secretion of FSTL-1 in skeletal muscle and
its implications for cardiac rehabilitation post-MI or post-op represent another intriguing
avenue for future exploration. By capitalizing on synergies between diverse therapeutic
modalities and integrating novel insights from related fields, we can unlock new dimensions
in cardiac regeneration and pave the way for transformative advancements in cardiovascular
medicine.
However, despite the overall agreement on FSTL-1's beneficial effects in cardiac regeneration,
there are notable discrepancies and contradictions in some areas of the literature.
For instance, while some studies demonstrate a clear cardioprotective role for FSTL-133, others suggest no significant impact on cardiac function when acting alone.44 Additionally, discrepancies exist regarding the mechanisms underlying FSTL-1 mediated
cardiomyocyte proliferation, with some studies implying activation of specific signaling
pathways, such as AKT and ERK, while others propose alternative mechanisms.
Furthermore, contradictory findings are observed regarding the optimal timing and
dosage of FSTL-1 administration for maximal therapeutic efficacy. Some studies suggest
that early administration of FSTL-1 post-injury is more effective in promoting cardiac
regeneration33, while others advocate for delayed intervention to allow for resolution of inflammation
and scar formation before initiating regenerative processes.5 Similarly, discrepancies exist regarding the source of FSTL-1 secretion and its precise
cellular targets within the myocardium, highlighting the need for further investigation
to elucidate these aspects.
Despite these advances in understanding FSTL-1, it is important to note that, as of
now, there are no clinical trials involving FSTL-1 in cardiac regeneration. However,
the future setup of such trials could involve carefully designed, phased approaches
to assess safety, dosage, timing, and efficacy. Initial trials might focus on determining
the optimal delivery method of FSTL-1, such as direct injection into the myocardium
or incorporation into biomaterials used in cardiac patches. These trials could also
investigate the timing of administration relative to myocardial infarction or surgical
intervention, as well as the integration of FSTL-1 with other therapies, such as stem
cell treatments or pharmacological agents. Subsequent trials would likely expand to
larger populations and longer follow-up periods to fully assess long-term outcomes
and potential benefits in preventing heart failure or improving functional recovery
post-MI. As research progresses and a deeper understanding of FSTL-1's role in cardiac
regeneration emerges, clinical trials will play a pivotal role in translating these
findings into viable therapeutic strategies.
Conclusion
The key findings from our review underscore the significant role of FSTL-1 as a stable
and effective cardio-protective and regenerative factor in cardiovascular research.
FSTL-1's multifaceted mechanisms demonstrate considerable potential in addressing
critical clinical challenges in MI treatment, such as the clearance of necrotic tissue,
restoration of lost cardiomyocytes, regeneration of electrical conductivity, and resolution
of inflammation and fibrotic tissue accumulation. Notably, our review highlights the
importance of optimizing FSTL-1 delivery methods—whether through intravenous administration
or via a nanofibrillar collagen patch—both of which hold promise for enhancing the
efficacy of therapeutic interventions and improving the quality of engrafted cells.
In the broader context of cardiovascular therapy, FSTL-1 emerges as a highly promising
therapeutic target with the potential to revolutionize clinical approaches to MI and
other cardiac conditions. The elucidation of FSTL-1's molecular mechanisms provides
a foundation for its integration into innovative treatment strategies, potentially
transforming current practices in cardiac care. Clinically, FSTL-1 offers avenues
for developing targeted therapies that could mitigate heart damage, promote tissue
regeneration, and improve long-term outcomes for patients. The versatility of FSTL-1
in addressing multiple facets of cardiac injury suggests that it could be pivotal
in creating personalized and precision-based treatments, particularly in high-risk
patient populations.
As research continues to delve into the complexities of FSTL-1's actions and refine
its delivery systems, the potential for FSTL-1 to serve as a cornerstone in cardiovascular
medicine becomes increasingly evident. With further development, FSTL-1 could lead
to groundbreaking therapies that significantly enhance patient recovery and survival
rates, marking a substantial advancement in the clinical management of MI and related
cardiac disorders.
Summary – Accelerating Translation
Follistatin-like 1 and its application in Ischaemic Heart Disease
FSTL-1 is a protein that has gained significant attention in the field of cardiac
rejuvenation due to its crucial role in promoting cardiac tissue repair and regeneration.
This study has as its main aim to identify, understand and summarise the molecular
and immune-physiological mechanisms underpinning this molecule and how it they might
apply to cardiac regeneration. Through this narrative review, the aim was to synthesise
information from various sources on this topic in a descriptive and/or qualitative
manner, with a more flexible and holistic overview of the existing literature.
FSTL-1 has been identified as a key player in this subject because of its ability
to stimulate the growth and repair of heart muscle tissue. This is of great importance
because the heart has limited regenerative capacity, and injuries or diseases can
lead to irreversible damage. FSTL-1 has also been found to activate cardiac stem cells
and promote their differentiation into functional cardiac muscle cells (cardiomyocytes).
This process is vital for replenishing damaged or dead cells in the heart after injury,
such as a heart attack. FSTL-1 exhibits anti-inflammatory properties by modulating
the immune response. Inflammation is a major contributor to heart damage following
cardiac events. By reducing inflammation, FSTL-1 helps create a more favorable environment
for cardiac repair. Another critical aspect of cardiac regeneration is the formation
of new blood vessels (angiogenesis) to supply oxygen and nutrients to regenerating
tissue. FSTL-1 has been shown to promote angiogenesis in the heart, facilitating the
healing process. FSTL-1 may also help reduce fibrosis, the excessive formation of
scar tissue in the heart, which can impair cardiac function. By limiting fibrosis,
FSTL-1 aids in preserving and restoring the heart's contractile function.
Understanding the importance of FSTL-1 in cardiac regeneration holds significant promise
for the development of novel therapeutic approaches for heart disease treatment. Researchers
are exploring ways to harness the regenerative potential of FSTL-1, such as through
gene therapy or the development of pharmaceutical agents that mimic its effects.
Acknowledgments
My thanks to Dr. Mário Santos, as my mentor and experienced cardiologist, he had the
chance to do a review of the overall manuscript.
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions
Conceptualization: JRG. Investigation: JRG. Writing - Review Editing: JRG.
References
1. Rørholm Pedersen L, Prescott E, Kerins M. ESC prevention of CVD Programme: Epidemiology of IHD. ESC Prevention of CVD Programme: Epidemiology of IHD. 2017;12(6):494–502.
2. du Pré BC, Doevendans PA, van Laake LW. Stem cells for cardiac repair: an introduction. J Geriatr Cardiol. 2020;17(2):1–5.
3. Daneshi N, Bahmaie N, Esmaeilzadeh A. Cell-Free Treatments: A New Generation of Targeted Therapies for Treatment of Ischemic
Heart Disease. 2021.
4. Kim Y, Zharkinbekov Z, Sarsenova M, Yeltay G, Saparov A. Recent Advances in Gene Therapy for Cardiac Tissue Regeneration. Int J Mol Sci. 2022;23(3):1025.
5. Vasu S, Zhou J, Chen J, Johnston PV, Kim DH. Biomaterials-based Approaches for Cardiac Regeneration. Korean Circ J. 2020;50(6):498–506.
6. Peters MC, Di Martino S, Boelens T, Qin J, van Mil A, Doevendans PA, et al. Follistatin-like 1 promotes proliferation of matured human hypoxic iPSC-cardiomyocytes
and is secreted by cardiac fibroblasts. Mol Ther Methods Clin Dev. 2019;14:93–105.
7. Ojha N, Dhamoon AS. Myocardial Infarction. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
8. Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when
is enough enough? Circulation. 2003;108(11):1395–403.
9. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilises two monocyte subsets with divergent
and complementary functions. J Exp Med. 2007;204(12):3037–47.
10. Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E, et al. Enhancing repair of the mammalian heart. Circ Res. 2007;100(11):1733–6.
11. Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced
proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111(2):743–55.
12. Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20(3):334–47.
13. Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function
and revascularisation in ischemic tissue through a nitric-oxide synthase-dependent
mechanism. 2008.
14. Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical
models. Circulation. 2012;126(14):1728–38.
15. Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury
to heart failure. Cardiovasc Res. 2011;90(2):224–33.
16. Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel E, et al. Follistatin-Like 1 Is an Akt-Regulated Cardioprotective Factor That Is Secreted by
the Heart. Circulation. 2008;117(24):3099–108.
17. Xi Y, Gong D-W, Tian Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial
Infarction Rats. Sci Rep. 2016;6:32445.
18. Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced
apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated
perfused heart. Circ Res. 2000;86(8):927–34.
19. Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic
mice. EMBO J. 2000;19(23):6341–50.
20. Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial
deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77(3):463–70.
21. Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13(12):1467–75.
22. Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, et al. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from
rupture. EMBO Mol Med. 2016;8(8):949–66.
23. Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress
induces monocyte adhesion by stimulating reactive oxygen species production from a
nox1-based NADPH oxidase. Circ Res. 2004;95(8):773–9.
24. Geng Y, Dong Y, Yu M, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist
in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108(17):7058–63.
25. Formigli L, Manneschi LI, Nediani C, Marcelli E, Fratini G, Orlandini SZ, et al. Are macrophages involved in early myocardial reperfusion injury? Ann Thorac Surg. 2000;70(6):1969–74.
26. Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World J Cardiol. 2010;2(11):345–52.
27. Pachori AS, Custer L, Hansen D, Clapp S, Kemppa E, Klingensmith J. Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent
signaling pathway. J Mol Cell Cardiol. 2010;48(6):1255–62.
28. Formigli L, Manneschi LI, Nediani C, Marcelli E, Fratini G, Orlandini SZ, et al. Are macrophages involved in early myocardial reperfusion injury? Ann Thorac Surg. 2000;70(6):1969–74.
29. Zuidema MY, Zhang C. Ischemia/reperfusion injury: The role of immune cells. World J Cardiol. 2010;2(11):345–52.
30. Shen H, Cui G, Li Y, et al. Follistatin-like 1 protects mesenchymal stem cells from hypoxic damage and enhances
their therapeutic efficacy in a mouse myocardial infarction model. Stem Cell Res. 2012;9(2):140–52.
31. Van Wijk B, Gunst QD, Moorman AFM, et al. Cardiac Regeneration from Activated Epicardium. PLoS One. 2012;7(10):e45626.
32. Shimano M, Ouchi N, Nakamura K, Van Wijk B, Ohashi K, Asaumi Y, et al. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure
overload. Proc Natl Acad Sci U S A. 2011;108(29):11727–32.
33. Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–85.
34. Chen W, Xia J, Hu P, Zhou F, Chen Y, Wu J, et al. Follistatin-like 1 protects cardiomyoblasts from injury induced by sodium nitroprusside
through modulating Akt and Smad1/5/9 signaling. Biochem Biophys Res Commun. 2015;460(2):259–65.
35. Pérez-Pomares JM, De La Pompa JL. Signaling During Epicardium and Coronary Vessel Development. Circ Res. 2011;109(12):1421–35.
36. Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac
regeneration and repair. Cell. 2013;154(4):827–42.
37. Hambrock HO, Kaufmann B, Muller S, Hanisch FG, Nose K, Paulsson M, et al. Structural characterisation of TSC-36/Flik: analysis of two charge isoforms. J Biol Chem. 2004;279(11):11727–35.
38. Rossdeutsch A, Smart N, Dubé KN, Turner M, Riley PR. Essential role for thymosin β4 in regulating vascular smooth muscle cell development
and vessel wall stability. Circ Res. 2012;111(8):e89–e102.
39. Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, et al. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from
rupture. EMBO Mol Med. 2016;8(8):949–66.
40. Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474(7353):640–4.
41. Shrivastava S, Srivastava D, Olson EN, DiMaio JM, Bock-Marquette I. Thymosin beta4 and cardiac repair. Ann N Y Acad Sci. 2010;1194:87–96.
42. Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454(7200):109–13.
43. Cao J, Poss KD. The epicardium as a hub for heart regeneration. Nat Rev Cardiol. 2018;15(10):631–47.
44. Görgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J. Regulation of follistatin-like protein 1 expression and secretion in primary human
skeletal muscle cells. Arch Physiol Biochem. 2013;119(1):1–8.
45. Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, et al. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular
injury. Cardiovasc Res. 2011;91(4):516–24.
46. Xi Y, Gong DW, Tian Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial
Infarction Rats. Sci Rep. 2015;5:15524.
47. Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, et al. CTRP9 Protein Protects against Myocardial Injury following Ischemia-Reperfusion through
AMP-activated Protein Kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287(22):18965–73.
48. Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in
myocardial infarction. Stem Cell Res Ther. 2017;8:242.
49. Lu L, Ma J, Liu Y, Shao Y, Xiong X, Duan W, et al. FSTL1-USP10-Notch1 Signaling Axis Protects Against Cardiac Dysfunction Through Inhibition
of Myocardial Fibrosis in Diabetic Mice. Front Cell Dev Biol. 2020;8:192.
José Rodrigues Gomes, 1 Fifth-year medical student at the School of Medicine and Biomedical Sciences Abel
Salazar, University of Porto, Portugal.
About the Author: I'm José Rodrigues Gomes, a fourth-year medical student at the School of Medicine
and Biomedical Sciences Abel Salazar, University of Porto. Currently, I am a Junior
Researcher at the Unit for Multidisciplinary Biomedical. My academic focus lies in
cardiovascular physiology and pathology, with a particular interest in their association
to obesity. Beyond research, I'm passionate about medical education, healthcare management
& ecnonomics, and sustainable clinical practices, envisioning a future where healthcare
aligns with ecological responsibility.
Correspondence: José Gomes. Address: R. Jorge de Viterbo Ferreira 228, 4050-313 Porto, Portugal.
Email: josemanuelrgomes@gmail.com
Editor: Francisco J. Bonilla-Escobar;
Student Editors:Himal Karki & Sayan Sarkar;
Proofreader: Amy Phelan;
Layout Editor: Julian A. Zapata-Rios;
Process: Peer-reviewed
Cite as
Gomes J. A Narrative Review on the FSTL-1 Protein and its Current Known Impact on
Cardiovascular Ischaemic Disease. Int J Med Stud. 2024 Oct-Dec;12(4):457-464.
Copyright © 2024 José Rodrigues Gomes
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 12, NUMBER 4, December 2024
