PRISMA Flowchart.
Felix Margenfeld1, Adib Zendehdel1, Giorgio Tamborrini2, Jennifer Polzer1, Marc Naville3, Amélie Poilliot4, Magdalena Müller-Gerbl1
doi: http://dx.doi.org/ijms.2024.2406
Volume 12, Number 3: 294-302
Received 11 10 2023; Rev-request 17 11 2023; Rev-request 09 07 2024; Rev-recd 21 11 2023; Rev-recd 12 07 2024; Accepted 14 07 2024
ABSTRACT
Bursitis is a common condition in clinical practice, often causing pain in the shoulder and buttock areas due to inflamed bursae. Proper diagnosis and treatment depend on knowing the presence and exact location of these bursae. Anatomy classes typically provide limited instruction on bursae because they are difficult to demonstrate during dissection courses. High-resolution ultrasound is an essential and versatile technique for detecting bursitis, and it could also serve as a valuable tool for students to better understand bursae. Relevant studies were screened in the following databases: CENTRAL, MEDLINE, BIOSIS Previews, EMBASE, and Web of Science Core Collection. Grey literature was also considered. Literature was screened on January 3, 2023. Only ultrasound investigations in human cadaver bursae were included, specifically using B-Mode ultrasound. The general characteristics of the included studies and the ultrasound-guided approaches for labeling the bursae were analyzed and examined. T The search found 8,899 matches, but only 15 met the criteria. Fifteen different bursae were studied, and 12 studies were included in the analysis. Both the marking substrate and the injected volume varied. Despite a high overall accuracy of 99% achieved using ultrasound-guided labeling approaches in the included studies, caution is advised due to the small sample size (1 to 24). The current study serves as a review to examine ultrasound studies on bursae in human cadavers. Ultrasound-guided labeling techniques achieve high accuracy and could be a valuable teaching tool in dissection courses. These techniques help visualize difficult-to-dissect structures and provide students with an understanding of sonoanatomy.
The first complete description of the bursae was published by Alexander Monro in 1788.1 Like sesamoid bones and tendon sheaths, bursae or bursas for the plural form, are extra-articular components. They present as thin, fluid-filled sacs that can sometimes communicate with the joint cavity2 or other nearby bursae3. There are two categories of bursae; native and non-native. Usually, the communicating bursae are a part of the native bursae. Native bursae are present from birth and are lined with a synovial membrane, therefore they are also called synovial bursae. Most commonly, they exist near large joints, and if they communicate with these joints, the synovial membranes (of the bursa and the joint) are continuous. Histologically, this synovial membrane consists of two layers4; one deep or outer layer and one superficial or inner layer. The inner lying cell layer produces a capillary film of synovial fluid on the inner surface of the sac, which acts as a lubricant. The deep vascularized layer is responsible for blood supply. Non-native bursae, which are also referred to as adventitious bursae, differ histologically from anatomical bursae (native bursae) because the synovial layer is absent5 and permeability is greater. As a result, hyaluronan and serum proteins can diffuse more easily.6
Depending on the position of both types of bursae, they are classified as subcutaneous, subtendinous, submuscular, or subfascial bursae and can be further named according to the location within the human body (e.g., subacromial, subscapular, ischiogluteal, trochanteric bursa).7 Bursae are classified as superficial (e.g., olecranon bursa) when they lie between bones, tendons or skin, and deep when they are between bones and muscles.7 Subcutaneous bursae are part of the superficial bursae and are often adventitious. They are created as a fusion of the superficial and deep fasciae, so they are a specialized form of fascia rather than a separate entity.8 The synovial fluid here is produced by specialized fibroblast-like cells, called fasciacytes, which also produce hyaluronan.9
All types of bursae have the function to reduce the friction that occurs during translation of the different tissues. Therefore, they are useful components in reducing tension and the negative effects of wear-and-tear at points of friction and provide resistance-free movement by the human body.10
In anatomy classes, bursae are taught in a limited fashion. This is because in the dissection course, bursae can rarely be shown, if at all in some cases. For bursae like the iliopectineal or the Pes Anserinus bursae, visualization can be successful with a careful approach. Students are often surprised at how much fluid leaks out which is a good indicator for a bursa when dissecting the area. Often, the iliopectineal bursa – which is connected to the hip joint - in particular shows a cartilaginous change as an expression of the pressure present in this area. Macroscopically, the surface of the bursa is shiny, highlighting its gliding ability. Other bursae such as the subdeltoid-subacromial or the bursae in the area of the greater trochanter are hardly visible. However, studies show that ultrasound-guided marking of the bursa on cadavers can be successful and thus, theoretically, may be useful in the visualization of bursae that are difficult for students to macroscopically observe during dissection courses.11 Technological advancements have led to significant developments. With the capacity for equipment to easily connect to tablets via Wi-Fi or Bluetooth, integrating ultrasound into medical student education has never been simpler. Consequently, students benefit doubly from early integration, as it enhances their anatomical knowledge and familiarizes them with ultrasound technology. Its benefit for clinical understanding is additionally enhanced.
Using modern ultrasound equipment with high resolution probes, superficial but also deep-seated bursae can be easily visualized. Many bursae are only visible when a pathology is present (e.g., bursitis iliopectinea), others are physiologically filled with fluid (e.g., bursa infrapatellaris profunda). We most frequently see clinically relevant bursitis in the shoulder and the greater trochanter12, where more than a dozen bursae exist.13 In the context of inflammatory bursitis, pain at rest and at night may occur (e.g., in the context of polymyalgia rheumatica of the hip or shoulders). In mechanically-induced bursitis, pain often occurs during movement. Some bursitis may also occur without pain (bursitis olecrani, bursitis prepatellaris). The etiology of bursitis varies. Causes can be systemic diseases such as rheumatoid arthritis, after vaccinations (shoulder injury related to vaccine administration = SIRVA), in the context of septic or infectious bursitis, in crystal arthritis, in the context of hydroxylapatite-associated bursitis, in large-vessel vasculitis (for example, in polymyalgia rheumatica), in the case of mechanical overload, after trauma or as accompanying symptoms of capsulitis.
The aim of the current paper is to provide an overview of the existing ultrasonographic observations and visualization methods on bursae of human cadavers found in the literature. The three specific objectives of this scoping review were to conduct a systematic search of the published and grey literature for ultrasonographic investigations on bursae of human cadavers, map out the key features and ultrasound-guided labeling techniques of the identified articles, and identify new research avenues with potential to advance the field.
The methodology followed the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines.14 The review included the following five key stages: (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data and (5) collating, summarizing, and reporting the results.
This review was guided by the question, “Is there evidence that ultrasound guided labeling techniques of bursae can be beneficial for learning anatomy in dissection courses?”. Therefore, a protocol was registered on November 28, 2022, on OSF Registries (osf.io/mx468).
The following databases were screened for relevant studies: CENTRAL, MEDLINE, BIOSIS Previews, EMBASE and Web of Science Core Collection. Grey literature was also considered in two different ways. (1) the grey literature database National Grey Literature Collection was regarded, (2) for PhD theses and dissertations, the databases EThOS and Open Access Theses and Dissertations were screened for relevant studies by combining the keywords used in the search strategy.
Backward and forward citation tracking was also performed. Search process was conducted on the 3rd of January 2023. The search strategy is attached below Supplementary Material 1. A librarian was contacted to develop the search strategy and reviewed the final version prior to use.
Following the search, all identified citations were collated using Endnote.15 Duplicates were removed by Endnote following Bramer, Giustini 16 and manually with further duplicates removed when found later in the review process. Title and abstract screening were also performed using Endnote.
Only ultrasound investigations in human cadavers were included. Since animal anatomy differs from human anatomy and Ultrasound in animals is still under-researched, these studies were excluded. Studies on phantoms were excluded due to the lower quality of ultrasound imaging.17 Other imaging techniques (MRI, CT) were not considered, primarily because ultrasound is distinguished as a low-radiation and resource-saving diagnostic instrument. It is portable, inexpensive and allows bedside examination. The article was included, if the investigated subject was a bursa. For the analysis of the ultrasound approaches only B-Mode ultrasound was included. Sonoeleastography was not used because actively perfused “living” tissue and tissue under a certain tension would be necessary (e.g. tense or relaxed tendon). At the present time, sonoelastography is not yet standardized and depends on the technique and the particular device. Doppler sonography makes little sense in cadavers. Since this work focuses on bursae, intravascular, intraosseous, intraarticular ultrasound was deliberately omitted. Only studies in English and German were included. In order to understand the exclusion criteria of the individual studies a PRISMA flowchart was created.
All types of relevant information including articles, PhD theses, dissertations and chapters in textbooks were considered. No restriction was placed on the year of publication.
First, title and abstract screening was performed for reviewing minimum inclusion criteria by one reviewer. References were added for full text screening if neither the title nor the abstract provided sufficient information. If uncertainties appeared, a second reviewer checked the references. Full text screening was performed by two reviewers. At each stage, disagreements were resolved by discussion or involvement of a third author. Anatomical structures were used to order the references. A critical appraisal of the included sources and a risk of bias assessment of the included studies were not performed. But for the publication bias, we checked whether the included studies registered a protocol.
Data were extracted from the papers included in the scoping review by one reviewer manually. General characteristics extracted included author, year of publication, title, investigated bursa and journal. The characteristics of ultrasound-guided labeling techniques that were extracted from the data comprised the marking substrate, injected volume, needle and sample size.
The data were further categorized in the citation manager Endnote and a spreadsheet was created and imported into Microsoft Excel 2019. Tables were created for the general characteristics and the ultrasound-guided labeling techniques. The articles were categorized by investigation approaches and presented in a narrative way.
One female 68-year-old formalin-fixed human specimen lying in supine position was used for the labeling of the iliopectineal bursa on the right side. Before injection a CT scan of the cadaver was performed and evaluated by a radiologist. No relevant pathologies were present. A sonographer with EULAR (https://www.eular.org/musculoskeletal-imaging-network-centres-list) certificate performed the ultrasound-guided injection with a linear probe (M12L, General Electric, model LOGIQ 9) using a standard in-plane technique.
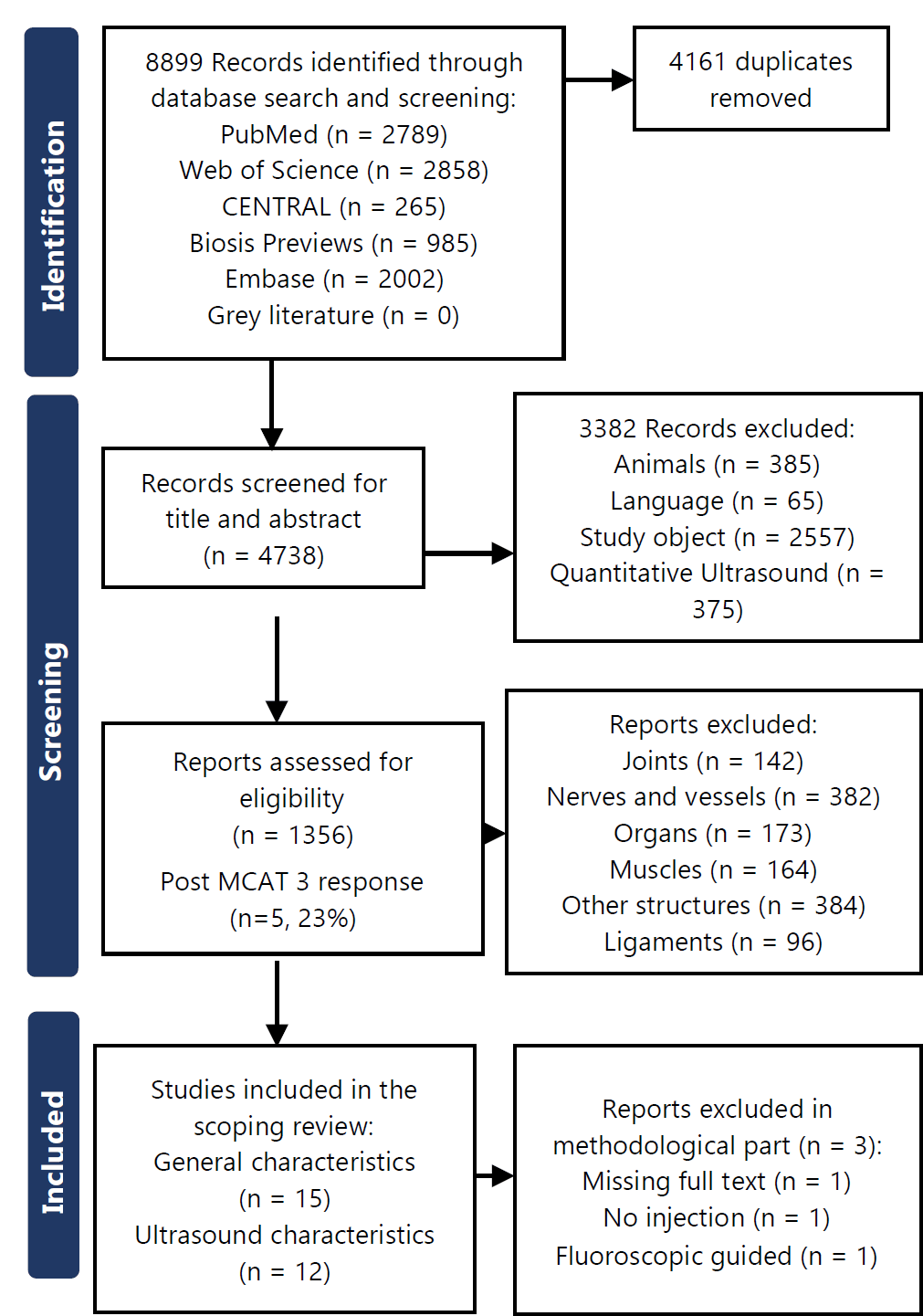
The search yielded 8899 results, of which 15 met the eligibility criteria. The authors agreed on all eligibility decisions upon discussion without the need for a third party to be involved. Forward and backward citation tracking of the 15 included publications did not yield any additional publications. The PRISMA flowchart is presented in Figure 1.
Figure 1.PRISMA Flowchart.

The general characteristics of the 15 studies are presented in Supplementary Material 2.
Seven of the included studies were published before 2015 and eight were from 2016 or earlier. Leading journals were AJR Am J Roentgenol and PM&R (both n = 3). All of the included studies were published in English. Only the subacromial bursa was investigated by two different studies.11,18 The following bursae were studied: the radial and ulnar bursae 19, the prepatellar bursa 20, the Pes Anserinus bursa 21, the Gruberi bursa 22, the subgluteus maximus and medius bursae 23, the trochanteric bursa 24, the medial collateral ligament bursa 25, the subdeltoid bursa 26, the semimembranosus bursa 27, the retrocalcaneal bursa 28, the subacromial bursa 11, the obturator internus bursa 29, the subacromial-subdeltoid bursa18, the infrapatellar bursa 30, and the ischial bursa31.
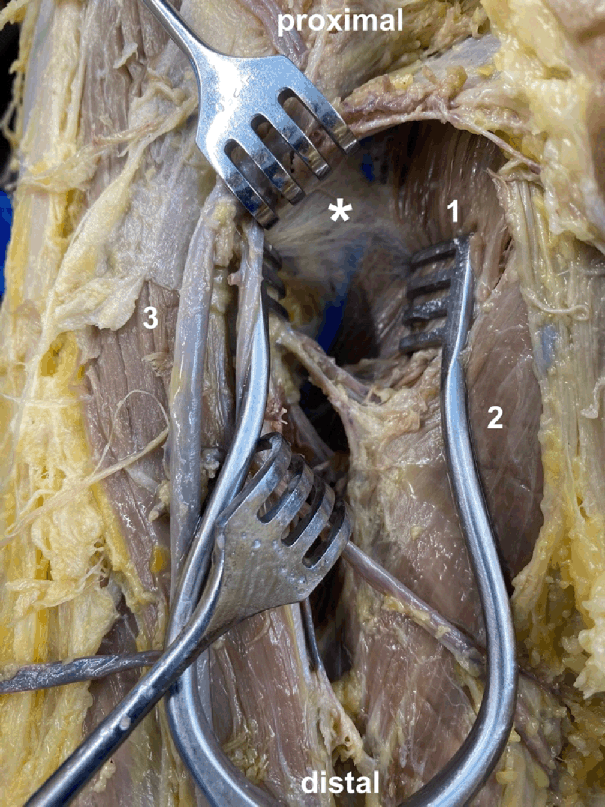
In the methodological analysis, 12 studies were included Table 1. Missing full text 23, no injection performed 26 and fluoroscopic guided bursography 18 were the main reasons why the three other studies were excluded. The sample sizes ranged from 1 to 24. Only in one study were some of the specimens formalin-embalmed 24, in all the other studies they were left non-embalmed. A latex solution had been applied in 6 investigations to label the bursae. The volumes of the latex solution ranged from 1.5 mL to 3 mL. Figure 2 shows an example staining of the iliopectineal bursa. Three studies performed a bursography via magnetic resonance imaging and therefore used a solution of gadopentate dimeglumine and other ingredients like iohexol or gelatin. They used volumes between 0.5 mL and 15 mL. One study injected, before the labeling substrate, a little amount of saline to spread the bursa.29 There were either 22-gauge or 25-gauge needles. The needle size was not addressed in four studies.19,25,28,30
Table 1.Summary of Ultrasound (US) Injection Characteristics of Included Studies (n = 12).
| Author (Year) | Structure | Sample characteristics | Substrate | Injection volume | Needle |
|---|---|---|---|---|---|
| Aguiar, Gasparetto 19 | Radial and ulnar bursae | Ten hands from nine fresh cadavers (five men and four women, mean age: 83.8 years), arms transected and immediately deep-frozen at −40 C; thaw for 24 hours at room temperature before Ultrasound | Solution consisting of 1 ml of gadopentate dimeglumine, diluted in 250 ml of saline solution and mixed with 0.5 ml of iohexol and 0.5 ml of a mixture of gelatin and different colors | 5–15 mL | 25-gauge needle |
| Aguiar, Viegas 20 | Prepatellar bursa | Nine knees from eight unembalmed cadavers (five women, four men, mean age: 76 years), transected and immediately deep-frozen at −40 C; thaw for 30 hours at room temperature before Ultrasound | Dilute gadolinium solution, two parts of 4 mmol/L gadopentate dimeglumine, one part of iodinated contrast material and one part of 15% concentrated solution of gelatin | 1–3 mL | n/c |
| Finnoff, Nutz 21 | Pes anserinus bursa | 24 unembalmed adult cadaveric lower extremity specimens; thawed at room temperature | Colored latex solution diluted by 50% with tap water | 2 mL | 25-gauge needle, 38-mm stainless steel needle |
| Gaetke-Udager, Jacobson 22 | Gruberi bursa | A single unembalmed cadaveric anklefoot specimen | Diluted blue latex (50% latex and 50% water) | 2 mL | 22-gauge needle |
| Mu, Peng 24 | Trochanteric bursa (Deep gluteus maximus bursa) | 24 hip specimens (10 male/14 female) from 12 cadavers (9 formalin-embalmed/3 fresh) with mean age of 79.5 years | Methylene blue | 1 mL | 22-gauge, 3.5-inch Quincke spinal needle |
| Nakase, Yoshimizu 25 | Medial collateral ligament bursa | Three fresh-frozen cadaver knees | Green ink | 1 mL | n/c |
| Onishi, Sellon 27 | Semimembranosus bursa | 10 unembalmed cadaveric knee specimens | Diluted blue-colored latex | 3 mL | 22-gauge, 63-mm, stainless steel needle |
| Pekala, Henry 28 | Retrocalcaneal bursa | 10 fresh-frozen specimens injected with ink 10 fresh-frozen specimens injected with iopromide All male, mean age 49.7 years, thawed for eight hours at room temperature prior to investigation |
|
2 mL | n/c |
| Pujalte, Hudspeth 11 | Subacromial bursa | 12 unembalmed cadaveric shoulder and complete upper extremity specimens (all males, with ages at death ranging from 40 to 50) | Combination of colored latex injection medium and uncompounded latex injection solution | 2–3 mL | 25-gauge, 38-mm stainless steel needle |
| Smith, Wisniewski 29 | Obturator internus bursa | 5 unembalmed cadaveric pelvis specimens, fresh-frozen, thawed at room temperature immediately before the study, mean age 78 years |
|
First small amount of saline than 1.5 mL diluted yellow latex | 22-gauge, 87.5-mm stainless steel needle |
| Viegas, Aguiar 30 | Deep and superficial infrapatellar bursae | Nine knee specimens from eight non-embalmed cadavers (five women, three men; mean age 76 years); immediately deep-frozen at −40 C; thaw for 30 h at room temperature | Dilute gadolinium solution (two parts of 4 mmol/l of gadopentate dimeglumine, one part iodinated contrast material, one part 15% concentrated solution of gelatin) | 0.5–1.5 mL | n/c |
| Wisniewski, Hurdle 31 | Ischial bursa | One unembalmed cadaveric pelvis | Diluted blue liquid latex (diluted by 50% with tap water | 3 mL | 22-gauge, 9-cm stainless-steel needle |
Staining of the Iliopectineal Bursa.
All three studies using MRI after bursography were able to successfully inject contrast agent into the investigated bursae with ultrasound guidance.19, 20, 30 With the help of the subsequent MRI, the extent of the bursae could be analyzed in detail. In the study by Aguiar, Gasparetto 19 for example, a communication between the radial and ulnar bursae could be found in every case. Furthermore, an “hourglass” or “figure of eight” shape with the constricting portion at the level of the carpal tunnel could be visualized. Aguiar, Viegas 20 saw a most frequently trilaminar (78%), followed by bilaminar (22%), anteriorly to the patella placed (= prepatellar) bursa. The average expansions were 3.2 mm (anteroposterior), 40.5 mm (lateromedial), and 39.7 mm (craniocaudal). Also, in the study by Viegas, Aguiar 30 the size and expansion of the deep and superficial infrapatellar bursae could be investigated. The average dimensions of deep infrapatellar bursa were as followed: craniocaudal 25 mm, mediolateral 28.7 mm and anteroposterior 6 mm. In 89% of the cases, it was subdivided into an anterior and posterior compartment. Lateral extension beyond the edge of the patellar tendon was observed in all specimens. Communication into the superficial bursa was observed once, but none communicated with the joint cavity of the knee. The edges of the superficial infrapatellar bursa could be defined in five specimens showing an average smaller size (craniocaudal 19.5 mm, mediolateral 21.2 mm and anteroposterior 2.2. mm).
Two studies compared ultrasound-guided versus landmark-guided approaches for bursae injection. Mu, Peng 24 injected methylene blue 12 times with ultrasound guidance and 12 times using landmark guidance into the subgluteus maximus bursa. The ultrasound-guided approach achieved 84% accuracy, the landmark-guided 60%. Finnoff, Nutz 21 also performed 12 ultrasound-guided and landmark-guided injection approaches into the Pes Anserinus bursa. Accuracies were 92% for ultrasound-guided and 17% for LM-guided injection. Three studies achieved an accuracy of 100% without overflow for the ultrasound-guided injection of the bursa which they investigated: Nakase, Yoshimizu 25 successfully targeted the medial collateral ligament bursa, Wisniewski, Hurdle 31 the ischial bursa on both sides, and Gaetke-Udager, Jacobson 22 the Gruberi bursa. Onishi, Sellon 27 injected latex with an accuracy of 100% by an overflow rate outside of the bursa of 80%. Pujalte, Hudspeth 11 injected the long head of the biceps tendon sheath successfully 12 times with concomitant subacromial bursa injection via the same needle. In two specimens (17%), the surrounding areas were penetrated by injectate. Smith, Wisniewski 29 included two different ultrasound-guided approaches into the obturator internus bursa. The two injections through the short-axis achieved an accuracy of 100% without overflow. One of the trans-tendinous injections was also completely effective, whereas the other achieved 80% of the injection with the remaining 20% in the obturator internus. Also the study by Pekala, Henry 28 had a high accuracy rate. In 10 fresh frozen Achilles tendon specimens, ink was injected into the retrocalcaneal bursa via ultrasound guidance. A similar pattern of ink spread could be found when comparing the radiographs that were made with contrast injections.
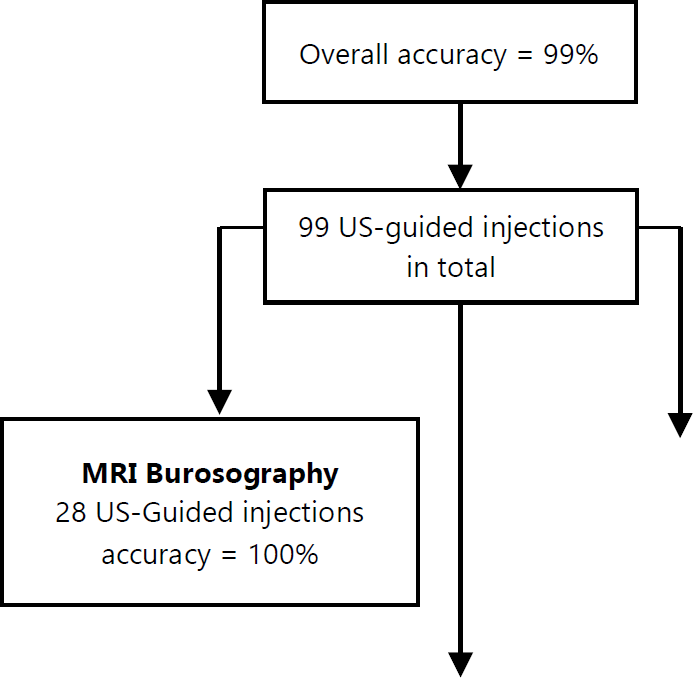
In total 99 ultrasound-guided injections into bursae were performed with an overall accuracy of 99%. Figure 3 summarizes the key findings.
Figure 3.Summary of the key findings.
This article represents a comprehensive review screening ultrasound investigations on bursae of cadavers. Reviews of ultrasound investigations on bursae of living objects are numerous. 32,33 Our study demonstrates the feasibility and viability of using ultrasound to conduct examinations on cadaveric bursae in multiple body regions. In this setting, bursae injections guided by ultrasound in cadavers are also distinguished by their great precision.11, 21, 27
In our view, and following a thorough exchange with the largest Universities in Switzerland - Zurich, Basel and Bern - learning about bursae tends to be less emphasized in anatomical educational institutions, particularly during the dissection course. This is not due to the anatomist's level of skill, but rather because the spaces are difficult to visualize macroscopically and thus difficult for students to understand. However, since bursae encompass a common etiology of pain syndromes, the present work could serve as an impetus to place more emphasis on teaching students about the importance of bursae. In this regard, the ultrasound-guided labeling technique may be an appropriate option. With the help of this method, bursae can be successfully labelled, and therefore students can better locate and observe them through this process. Learning with the help of colors seems to facilitate the memory consolidation process34 and implementing this does not require a lot of material. Even small amounts of a labelling substance (e.g., latex, methylene blue or ink; which are all equal); usually 1–3 mL are sufficient to label the bursa successfully with ultrasound-guidance.21, 24, 25 Furthermore, the use of ultrasound as a teaching tool allows for medical students to gain practical skills 35 and their clinical anatomical knowledge will be also be improved. We believe that this should be taught intensively in medical school and not after the end of training. The survey from O'Keeffe, Davy 36 to radiologists proves the assumption that this clinical-anatomical knowledge should be taught only as a combination. Ultrasound is flexible, inexpensive, and versatile. There are now portable probes with high resolution that can be easily connected to tablets or even smartphones via Bluetooth or WLAN. Protective covers for the tablets and ultrasound devices ensure cleanliness, even if the dissection is already at an advanced stage. Assuming approximately 8–10 students per dissection table, the practical application of ultrasound by the students themselves is also conceivable. Students could visualize difficult-to-dissect structures with the assistance of instructors trained in ultrasound. By using this tool already in the dissection course will set the students up for diagnostic and therapeutic skills and prove that the ultrasound is the next new pocket-sized stethoscope.37
The question that arises, however, is whether the injection techniques, which have been performed primarily on fresh-frozen body donations, can be transferred to the dissection course which commonly uses formalin-fixed body donations. Fresh frozen cadavers pose numerous challenges, including the requirement of freezers for storage and limited work time (a few weeks at the most) because of rapid decomposition following thawing. 38 Therefore, they are not suitable for a dissection course that typically spans several months. Formalin on the other hand hardens the tissues, thus its use is generally associated with extreme rigidity and has been found to severely affect the quality of cadaveric tissue, particularly soft tissues. 39 Only the study of Mu, Peng 24 included formalin fixed cadavers in addition to fresh frozen cadavers. Differences in the accuracy rate for ultrasound-guided trochanteric bursa injections between the two cadaver types was not reported. Further studies should be performed on formalin-fixed body donors to provide clarity. Thiel fixation could be an alternative. Eisma, Lamb 40 argue that Thiel-embalmed cadavers are advantageous, especially for teaching of the musculoskeletal system. However, they are more expensive than formalin-fixed cadavers, and the embalming procedure is more complex. 41 None of the included studies used Thiel preserved cadavers so it is unclear whether Thiel-fixed cadavers can better represent the bursae and whether they may be beneficial for teaching bursae anatomy. Bursae are among the most difficult structures to visualize in the dissection course, belong according to Stecco's work on the fasciae and are therefore largely influenced by the fixation method similar to fascial tissue. 42
In the current text, all processes were conducted with rigor and transparency. It followed a protocol that was listed in the OSF Registries. To provide a complete search of the literature, five digital bibliographical databases and a grey literature search were incorporated in the course of the investigation. Since the initial goal was to examine all ultrasound tests conducted on body donors, the search terms were purposefully broad. As a result, there were many studies returned by the search, which made the title and abstract screening process time-consuming and perhaps mistake-prone.
As mentioned above, most studies were conducted on fresh frozen cadavers. Therefore, the transferability of these results to formalin-fixed cadavers should be considered cautiously, as studies indicate that ultrasound quality and visibility of structures varies significantly between preservation methods.43 Further research is needed on formalin-fixed cadavers. Although high accuracy was achieved, some studies were conducted on only one cadaver with fewer than five injections performed. 22, 31
Scoping reviews do not adhere to the same strict standards that systematic reviews do, and there is no risk of bias assessment, which leaves space for biases like selection bias. Publication bias is strong since none of the included studies registered a visible protocol prior to their investigation. Generally, caution should be taken when drawing conclusions from scoping reviews because they frequently summarize the findings without fully synthesizing the results.
The present study evaluates the current literature on the bursae and acts as a comprehensive review to screen ultrasound studies of human cadaveric bursae. The ultrasound-guided labeling procedures produced bursae labels with a high degree of accuracy. Therefore, ultrasound as a versatile and portable instrument could be a potential teaching tool to visualize difficult-to-dissect structures such as bursae during the dissection course and provide students with an understanding of sonoanatomy. But, caution is advised in drawing general conclusions because of the small number of identified studies, small sample sizes and different methodologies in the studies. Future larger-scale research on different fixation methods (Formalin, Thiel) are necessary.
Ziel unserer Arbeit “Schleimbeutel im Präparationskurs – der Wert von Ultraschallbildgebungsverfahren zur Verbesserung der anatomischen Visualisierung und des Verständnisses der Studierenden” war es eine Übersicht von Ultraschalluntersuchungen an Schleimbeuteln von menschlichen Körperspendern zu generieren. Die Anatomie und klinische Bedeutung von Schleimbeuteln wird im Medizinstudium gelehrt, jedoch können Schleimbeutel im Präparationskurs, unter anderem aufgrund der Fixiationsmethode, nur schwer dargestellt und somit nur schwer von Studierenden nachvollzogen werden. Ultraschall ist in der Lage Schleimbeutel und deren Erkrankungen sicher am Lebenden nachzuweisen. Allerdings gibt es wenig Daten ob dies an Körperspendern auch möglich ist. Die weiterführende Idee dieser Arbeit war es die Daten zu Ultaschalluntersuchungen an Körperspendern systematisch zusammenzufassen, um Schlussfolgerungen für die Verbesserung der Lehre schliessen zu können. Die Leitfrage dabei war: Ist die frühe Integration des Ultraschalls in die medizinische Lehre für Medizinstudierende vorteilhaft?
Eine systematische Literaturrecherche wurde streng nach den PRISMA Vorgaben durchgeführt. 8899 Referenzen wurde gefunden, wovon sich 15 mit dem Thema Schleimbeutel befassten. Es zeigten sich unterschiedliche Ultraschallexperimente: Ultraschall gestützte Injektionen von Schleimbeuteln mit Kontrastmittel oder Latex wurden durchgeführt. Anschliessende Magnetresonanztomographie-oder Röntgen-Aufnahmen wurden erstellt. In den meisten Studien wurde eine Dissektion der Region durchgeführt. Eine hohe Präzision der Injektionen konnte in allen Studien gezeigt werden. Während der Dissektion konnten die Schleimbeutel durch die Anfärbung (bspw. mit Latex) sicher lokalisiert und nachvollzogen werden. Die Ergebnisse zeigen, dass der Ultraschall und Ultraschall gestützte Injektionen Schleimbeutel auch an Körperspendern sicher lokalisieren und darstellen können. Studierende würden von einer frühen Implementierung des Ultraschalls in den Präparationskurs zweifach profitieren: Sie würden einerseits den frühen Umgang mit Ultraschall erlernen und andererseits Ihr anatomisches Wissen vertiefen. Daher sprechen wir uns für eine frühe Integration des Ultraschalls während des Medizinstudiums aus.
None
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: FM, and AZ. Methodology: FM, and AZ. Investigation: FM, and GT. Resources: MM. Writing – Original Draft: FM. Writing – Review & Editing: AZ, GT, JP, MN, and AP. Visualization: FM, AZ, and AP. Supervision: MM. Project Administration: MM.
Supplementary Material Appendix 1.Search Strategy.| Database | Search term (translated with the aid of https://sr-accelerator.com/#/polyglot) |
|---|---|
| PubMed | (ultrasonography[mesh] OR ultrasonography[tiab] OR ultraso*[tiab]) AND (cadaver[mesh] OR cadaver*[tiab] OR corpse*[tiab] OR dead bod*[tiab] OR donated bod*[tiab] OR body donation*[tiab] OR deceased*[tiab] OR lifeless[tiab]) NOT (animals[mesh] NOT humans[mesh]) NOT (animal*[tiab] NOT human*[tiab]) NOT (transplantation[mesh]) NOT (transplantation[tiab]) |
| CENTRAL | (ultrasonography OR ultrasonography OR ultraso*) AND (cadaver OR cadaver* OR corpse* OR dead bod* OR donated bod* OR body donation* OR deceased* OR lifeless) NOT (animals NOT humans) NOT (animal* NOT human*) NOT (transplantation) |
| Embase | (‘ultrasonography’/exp OR ultrasonography:ti,ab) AND (‘cadaver’/exp OR cadaver*:ti,ab) NOT (‘animals’/exp NOT ‘humans’/exp) NOT (animal*:ti,ab NOT human*:ti,ab) NOT (‘transplantation’/exp) NOT (transplantation:ti,ab) |
| Web of Science | (ultrasonography OR ultrasonography OR ultraso*) AND (cadaver OR cadaver* OR corpse* OR “dead bod*” OR “donated bod*” OR “body donation*” OR deceased* OR lifeless) NOT (animals NOT humans) NOT (animal* NOT human*) NOT (transplantation) |
| Biosis Previews | (ultrasonography OR ultrasonography OR ultraso*) AND (cadaver OR cadaver* OR corpse* OR “dead bod*” OR “donated bod*” OR “body donation*” OR deceased* OR lifeless) NOT (animals NOT humans) NOT (animal* NOT human*) NOT (transplantation) |
| Author/Year | Title | Bursa/e | Journal |
|---|---|---|---|
| Aguiar, Gasparetto 19 | Radial and ulnar bursae of the wrist: cadaveric investigation of regional anatomy with ultrasonographic-guided tenography and MR imaging | Radial and ulnar bursae | Skeletal Radiol |
| Aguiar, Viegas 20 | The prepatellar bursa: cadaveric investigation of regional anatomy with MRI after sonographically guided bursography | Prepatellar bursa | AJR Am J Roentgenol |
| Finnoff, Nutz 21 | Accuracy of Ultrasound-Guided versus Unguided Pes Anserinus Bursa Injections | Pes anserinus bursa | PM&R |
| Gaetke-Udager, Jacobson 22 | Ultrasound of the Gruberi Bursa With Cadaveric and MRI Correlation | Gruberi bursa | AJR Am J Roentgenol |
| Moore, Johnson 23 | Distribution of Sonographically Guided Injections of the Subgluteus Minimus and Medius Bursae in Cadaveric Model | Subgluteus and medius bursae | Med Sci Sports Exerc |
| Mu, Peng 24 | Landmark-Guided and Ultrasound-Guided Approaches for Trochanteric Bursa Injection: A Cadaveric Study | Trochanteric bursa | Anesth Analg |
| Nakase, Yoshimizu 25 | Anatomical description and short-term follow up clinical results for ultrasound-guided injection of medial collateral ligament bursa: New conservative treatment option for symptomatic degenerative medial meniscus tear | Medial collateral ligament bursa | Knee |
| Norbury, Karr 26 | Improving the Performance Time and Accuracy of Ultrasound-Guided Interventions: A Randomized Controlled Double-Blind Trial of the Line-of-Sight Approach and the “APPLES” Mnemonic | Subdeltoid bursa | J Ultrasound Med |
| Onishi, Sellon 27 | Sonographically Guided Semimembranosus Bursa Injection: Technique and Validation | Semimembranosus bursa | PM&R |
| Pekala, Henry 28 | The Achilles tendon and the retrocalcaneal bursa: an anatomical and radiological study | Retrocalcaneal bursa | Bone Jt Res |
| Pujalte, Hudspeth 11 | Ultrasound-guided injection of the long head of the biceps tendon sheath with concomitant subacromial bursa injection through the same needlestick | Subacromial bursa | Clin Anat |
| Smith, Wisniewski 29 | Sonographically guided obturator internus injections: techniques and validation | Obturator internus bursa | J Ultrasound Med |
| Stallenberg, Destate 18 | Involvement of the anterior portion of the subacromial-subdeltoid bursa in the painful shoulder | Subacromial-subdeltoid bursa | AJR Am J Roentgenol |
| Viegas, Aguiar 30 | Deep and superficial infrapatellar bursae: cadaveric investigation of regional anatomy using magnetic resonance after ultrasound-guided bursography | Infrapatellar bursa | Skeletal Radiol |
| Wisniewski, Hurdle 31 | Ultrasound-guided Ischial Bursa Injection: Technique and Positioning Considerations | Ischial bursa | PM&R |
1. Monro A. A description of all the bursae mucosae of the human body; their structure explained, and compared with that of the capsular ligaments of the joints, and of those sacs which line the cavities of the thorax and abdomen; with remarks on the accidents and diseases which affect those several sacs, and on the operations necessary for their cure. Edinburgh: Printed for C. Elliot, T. Kay …, London; and for Charles Elliot, Edinburgh; 1788.
2. Koudela K, Jr., Koudelová J, Koudela K, Sr., Kunesová M. Bursitis iliopectinea. Acta Chir Orthop Traumatol Cech. 2008;75(5):347–54.
3. Dihlmann W, Peters A, Tillmann B. The bursa iliopectinea—a morphologic-computed tomographic study. Rofo. 1989;150(3):274–9.
4. Resnick DLK, H. S.; Pretterklieber, M.L. Internal derangements of joints. 2nd ed. Philadelphia: Saunders; 2007.
5. Canoso JJ, Stack MT, Brandt KD. Hyaluronic acid content of deep and subcutaneous bursae of man. Ann Rheum Dis. 1983;42(2):171–5.
6. Van Mieghem IM, Boets A, Sciot R, Van Breuseghem I. Ischiogluteal bursitis: an uncommon type of bursitis. Skeletal Radiol. 2004;33(7):413–6.
7. Sawyer E, Varacallo M. Anatomy, shoulder and upper limb, hand ulnar bursa. StatPearls. Treasure Island (FL): StatPearls Publishing, Copyright © 2023, StatPearls Publishing LLC.; 2023.
8. Stecco C. Atlas des menschlichen fasziensystems: Elsevier Health Sciences; 2016.
9. Stecco C, Fede C, Macchi V, Porzionato A, Petrelli L, Biz C, et al The fasciacytes: A new cell devoted to fascial gliding regulation. Clin Anat. 2018;31(5):667–76.
10. De Oliveira-Lagôa S, Cruz FB, Azócar DLM, Lavilla EO, Abdala V. Anuran forelimb muscle tendinous structures and their relationship with locomotor modes and habitat use. Curr Zool. 2019;65(5):599–608.
11. Pujalte G, Hudspeth LJ, Troyer WD, Shapiro SA. Ultrasound-guided injection of the long head of the biceps tendon sheath with concomitant subacromial bursa injection through the same needlestick. Clin Anat. 2022.
12. Tamborrini G, Marx C. Cme-rheumatology 7: trochanter major pain syndrome. Praxis (Bern 1994). 2016;105(3):172–4.
13. Tamborrini G, Müller-Gerbl M, Müller SA. Cme-sonography 106: subacromial bursa - a myth. Praxis (Bern 1994). 2022;111(15):833–46.
14. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73.
15. Gotschall T. EndNote 20 desktop version. J Med Libr Assoc. 2021;109(3):520–2.
16. Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–3.
17. Dang D, Kamal M, Kumar M, Paliwal B, Nayyar A, Bhatia P, et al Comparison of human cadaver and blue phantom for teaching ultrasound-guided regional anesthesia to novice postgraduate students of anesthesiology: a randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2024;40(2):276–82.
18. Stallenberg B, Destate N, Feipel V, Gevenois PA. Involvement of the anterior portion of the subacromial-subdeltoid bursa in the painful shoulder. AJR Am J Roentgenol. 2006;187(4):894–900.
19. Aguiar RO, Gasparetto EL, Escuissato DL, Marchiori E, Trudell DJ, Haghighi P, et al Radial and ulnar bursae of the wrist: cadaveric investigation of regional anatomy with ultrasonographic-guided tenography and MR imaging. Skeletal Radiol. 2006;35(11):828–32.
20. Aguiar RO, Viegas FC, Fernandez RY, Trudell D, Haghighi P, Resnick D. The prepatellar bursa: cadaveric investigation of regional anatomy with mri after sonographically guided bursography. AJR Am J Roentgenol. 2007;188(4):W355–8.
21. Finnoff JT, Nutz DJ, Henning PT, Hollman JH, Smith J. Accuracy of ultrasound-guided versus unguided pes anserinus bursa injections. PM&R. 2010;2(8):732–9.
22. Gaetke-Udager K, Jacobson JA, Bhatti ZS, Smith J, Parameswaran A, Fessell DP. Ultrasound of the gruberi bursa with cadaveric and mri correlation. AJR Am J Roentgenol. 2016;207(2):386–91.
23. Moore BJ, Johnson W, Finnoff JT, Smith J, Sellon JL. Distribution of sonographically guided injections of the subgluteus minimus and medius bursae in cadaveric model. Med Sci Sports Exerc. 2019;51(6, Suppl. S):592.
24. Mu A, Peng P, Agur A. Landmark-guided and ultrasound-guided approaches for trochanteric bursa injection: a cadaveric study. Anesth Analg. 2017;124(3):966–71.
25. Nakase J, Yoshimizu R, Kimura M, Kanayama T, Yanatori Y, Tsuchiya H. Anatomical description and short-term follow up clinical results for ultrasound-guided injection of medial collateral ligament bursa: New conservative treatment option for symptomatic degenerative medial meniscus tear. Knee. 2022;38:1–8.
26. Norbury JW, Karr NC, Sindhi V, Rathbun KM, Charles SC, McIver MB, et al Improving the performance time and accuracy of ultrasound-guided interventions: a randomized controlled double-blind trial of the line-of-sight approach and the “apples” mnemonic. J Ultrasound Med. 2018;37(12):2909–14.
27. Onishi K, Sellon JL, Smith J. Sonographically guided semimembranosus bursa injection: technique and validation. PM&R. 2016;8(1):51–7.
28. Pekala PA, Henry BM, Pekala JR, Piska K, Tomaszewski KA. The Achilles tendon and the retrocalcaneal bursa an anatomical and radiological study. Bone Jt Res. 2017;6(7):446–51.
29. Smith J, Wisniewski SJ, Wempe MK, Landry BW, Sellon JL. Sonographically guided obturator internus injections: techniques and validation. J Ultrasound Med. 2012;31(10):1597–608.
30. Viegas FC, Aguiar RO, Gasparetto E, Marchiori E, Trudell DJ, Haghighi P, et al Deep and superficial infrapatellar bursae: cadaveric investigation of regional anatomy using magnetic resonance after ultrasound-guided bursography. Skeletal Radiol. 2007;36(1):41–6.
31. Wisniewski SJ, Hurdle M, Erickson JM, Finnoff JT, Smith J. Ultrasound-guided ischial bursa injection: technique and positioning considerations. PM&R. 2014;6(1):56–60.
32. Wu T, Song HX, Dong Y, Li JH. Ultrasound-guided versus blind subacromial-subdeltoid bursa injection in adults with shoulder pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;45(3):374–8.
33. McGill KC, Patel R, Chen D, Okwelogu N. Ultrasound-guided bursal injections. Skeletal Radiol. 2023;52(5):967–78.
34. Dzulkifli MA, Mustafar MF. The influence of colour on memory performance: a review. Malays J Med Sci. 2013;20(2):3–9.
35. Hamza A, Radosa J, Meyberg-Solomayer G, Solomayer EF, Takacs Z, Juhasz-Boess I, et al Trial integration of combined ultrasound and laparoscopy tuition in an undergraduate anatomy class with volunteer participation - a pilot study. Ann Anat. 2019;221:101–7.
36. O'Keeffe GW, Davy S, Barry DS. Radiologist's views on anatomical knowledge amongst junior doctors and the teaching of anatomy in medical curricula. Ann Anat. 2019;223:70–6.
37. Bledsoe A, Zimmerman J. Ultrasound: the new stethoscope (point-of-care ultrasound). Anesthesiol Clin. 2021;39(3):537–53.
38. Hayashi S, Naito M, Kawata S, Qu N, Hatayama N, Hirai S, et al History and future of human cadaver preservation for surgical training: from formalin to saturated salt solution method. Anat Sci Int. 2016;91(1):1–7.
39. Wilke HJ, Werner K, Häussler K, Reinehr M, Böckers TM. Thiel-fixation preserves the non-linear load-deformation characteristic of spinal motion segments, but increases their flexibility. J Mech Behav Biomed Mater. 2011;4(8):2133–7.
40. Eisma R, Lamb C, Soames RW. From formalin to thiel embalming: what changes? One anatomy department's experiences. Clin Anat. 2013;26(5):564–71.
41. Wolff KD, Kesting M, Mücke T, Rau A, Hölzle F. Thiel embalming technique: a valuable method for microvascular exercise and teaching of flap raising. Microsurgery. 2008;28(4):273–8.
42. Stecco C, Fantoni I, Macchi V, Del Borrello M, Porzionato A, Biz C, et al The role of fasciae in civinini-morton's syndrome. J Anat. 2015;227(5):654–64.
43. Sawhney C, Lalwani S, Ray BR, Sinha S, Kumar A. Benefits and Pitfalls of Cadavers as Learning Tool for Ultrasound-guided Regional Anesthesia. Anesth Essays Res. 2017;11(1):3–6.
Felix Margenfeld, 1 MD. Institute of Anatomy, University of Basel, Switzerland.
Adib Zendehdel, 1 MD. Institute of Anatomy, University of Basel, Switzerland.
Giorgio Tamborrini, 2 MD. Rheumatology Clinic, University Hospital of Basel, Switzerland.
Jennifer Polzer, 1 MD. Institute of Anatomy, University of Basel, Switzerland.
Marc Naville, 3 MD. University of Zurich, Faculty of Medicine, Zurich, Switzerland.
Amélie Poilliot, 4 PhD. Institute of Anatomy, University of Basel, Switzerland.
Magdalena Müller-Gerbl, 1 MD. Institute of Anatomy, University of Basel, Switzerland.
About the Author: Marc Naville is a recently graduated physician working in an orthopedic department near Zurich.
Correspondence: Marc Naville. Address: Pestalozzistrasse 3 8032 Zurich, Switzerland. Email: felix.margenfeld@unibas.ch
Editor: Francisco J. Bonilla-Escobar; Student Editors: Marco Becciolini & Ondřej Naňka; Proofreader: Amy Phelan; Layout Editor: Julian A. Zapata-RiosSubmission: Oct 11, 2023; Process: Peer-reviewed
Cite as Margenfeld F, Zendehdel A, Tamborrini G, Polzer J, Naville M, Poilliot A, et al. A Scoping Review on the Utility of Ultrasound to Visualize Bursae in Anatomical Dissection Courses. Int J Med Stud. 2024 Jul-Sep;12(3):294-302.
Copyright © 2024 Felix Margenfeld, Adib Zendehdel, Giorgio Tamborrini, Jennifer Polzer, Marc Naville, Amélie Poilliot, Magdalena Müller-Gerbl
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 12, NUMBER 3, September 2024