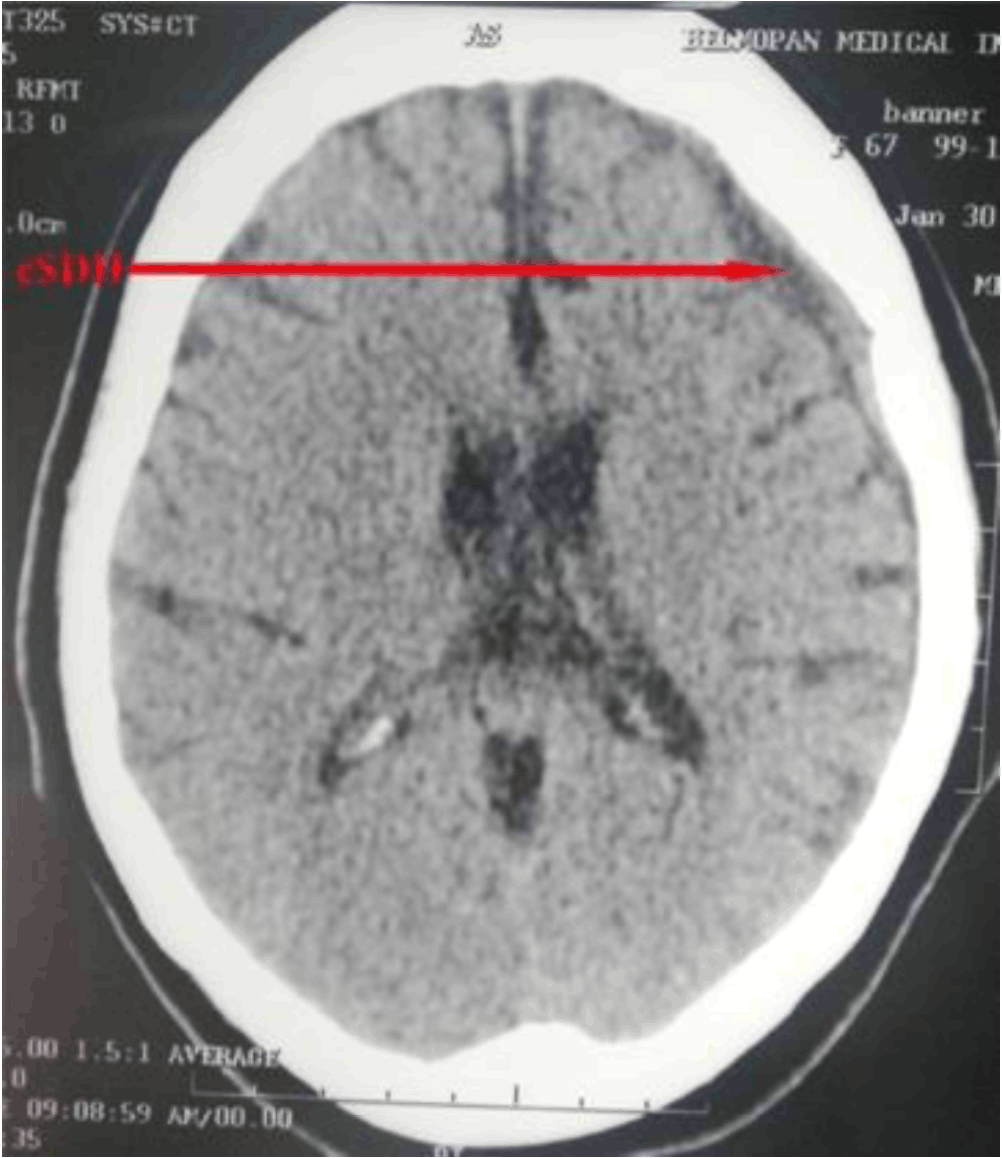
Axial non-contrast CT image showing a crescent shaped isodense subdural hematoma in the left hemisphere of the parietal lobe. The right hemisphere, ventricular system, and deep structures of the brain appear normal with no midline shift.
doi: http://dx.doi.org/10.5195/ijms.2019.371
Volume 7, Number 2: 38-40
Received 02 04 2019: Accepted 09 08 2019
ABSTRACT
Background:Chronic Subdural Hematoma (CSDH) is becoming an urgent public health issue due to an increase of incidence in aging populations like Taiwan. Though trauma still stands as the primary mechanism of CSDH, it is often overlooked in the elderly, especially those with mid-to-late stage Alzheimer's Disease (AD). Coincidentally, the clinical presentation of mid-to-late stage AD shares significant overlap with CSDH. AD creates an immense challenge for physicians and family members to identify early signs of CSDH.
The Case:We report a peculiar case of a 67-year-old female with a history of AD who presents to the Emergency room in Belmopan City, Belize, with recurrent CSDH. On admission her consciousness was disturbed and late stage dementia presented an enormous challenge for logical and meaningful history taking. Axial non-contrast computed tomography showed a crescent-shaped isodense subdural hematoma in the left hemisphere of the parietal lobe. She was stabilized and treated conservatively with corticosteroids, beta blockers, angiotensin-converting enzyme (ACE) inhibitors and diuretics.
Conclusion:It is important for physicians to recognize and develop protocols to identify early signs of CSDH in patients with late stage AD. Early management is a key factor in minimizing more serious complications like recurrence, seizures, and tension pneumocephalus.
Keywords: Chronic Subdural Hematoma; Alzheimer Disease; Trephining (Source: MeSH-NLM)..
Chronic subdural hematoma (CSDH) is one of the most common neurosurgical conditions in which blood accumulates between the arachnoid mater and the dura mater.3-7 The world wide annual incidence of CSDH is estimated at 8.2 per 100 000 per year in people above the age of 65.1,8 CSDH is becoming a more urgent public health issue due to an increase in incidence in aging populations such as in Taiwan.1
CSDH is a slow accrual of encapsulated fluid containing blood and degradation products that sit between the dura mater and the arachnoid mater. 3, 7 CSDH is predominant among the elderly and a long standing theory suggests that head trauma is the primary risk factor - accounting for over 70% of cases.1,7,8 Vascular malformations, brain tumors, alcoholism and seizures are also significant predisposing factors that should also be taken into consideration. 7 Recently though, the trauma theory has been subjected to intense scrutiny as it takes roughly 4 to 7 weeks following a head injury for a CSDH to become symptomatica. 7 However, this time frame has some inconsistencies as even a slow venous hemorrhage would accumulate quickly enough to become symptomatic within just a few days.7,9
The presentation of CSDH varies significantly from no symptoms to headache, seizures, decreased memory, and confusion.1, 3, 5,7-9 Coincidentally, the presentation of mid-to-late stage AD shares significant overlap with those of CSDH.2 This situation creates an immense challenge for physicians to identify early signs of CSDH and its re-occurrence in patients with concurrent AD. However, it must be considered that the majority of CSDHs are largely attributed to trauma, intracranial hypotension and defective coagulations.2,12
Inflammation, is known to play a crucial role in the development of CSDH.7 Despite its role in tissue repair, prolonged inflammatory responses directly contributes to fluid accumulation and angiogenesis leading to new membrane growth through CSDH.7 IL-6 and IL-8 have also been elucidated as important inflammatory cytokines implicated in fibrinolysis. IL-6 and IL-8 are produced by a host of cell types including monocytes, fibroblasts, and endothelial cells.7
The pathology of the enlargement of the CSDH is very complex but this condition is treated most effectively through surgery.7 Middle Meningeal Artery (MMA) embolization and burr hole evacuation are relatively simple procedures, recurrences remain one of the major bottlenecks in treatment.6,7,9
On January 29th, 2019 a 67-year-old female patient with a history of stage III hypertension, subdural hematoma, and late state dementia presented to the Department of Accident and Emergency at Western Regional Hospital in Belmopan City, Belize, due to consciousness disturbance, syncope, and left-sided hemiparesis. The symptoms were most pronounced on the day leading up to admission. On admission her consciousness was disturbed and late stage dementia presented an enormous challenge for logical and meaningful history taking. The patient's family denied the use of antiplatelet and anticoagulants. Her Glasgow Coma Scale was E4V4M5 (11). Vital signs in the ER revealed a temperature of 36. 1 °C, pulse rate of 61, respiratory rate of 14, oxygen saturation of 90%, and a blood pressure of 120/70. Fluid resuscitation was initiated and blood samples were taken. Prior to this second round of admission, she was admitted on January 1st 2019 for a burr hole craniotomy (trephination) to irrigate and drain a subdural hematoma in the left hemisphere of the parietal lobe.
ER workup revealed microcytic anemia with hemoglobin 10.8 g/dl (norm 13-18 g/dl), hemotocrit 32.1%, (norm 40-54%) and MCV 78 fl (norm 80-94 fl) without increased WBC (white blood cells). Glucose level were normal (106 mg/dl) and urine analysis was unremarkable. The CT was performed using a multidetector scanner Brivo CT325. Continuous helical slices of 5 mm thick sections across the entire neurocranium were performed. Reconstruction at 3 mm slices and 1.5 mm intervals were also obtained. The osseous tissues and pneumotized cavities were studied using bone window which revealed a recent left parietal burr hole.
The CT imaging revealed an extra axial crescent shape isodense collection affecting the left parietal region associated with effacement of the cerebral sulci in the left parietal lobe due to edema. The cerebellar hemispheres, vermis, brainstem and cerebellar-pontine angles appeared normal in density and contour. The ventricular system also appeared normal in size and position relative to the midline. Infratentorial and supratentorial cisternae and the external subarachnoid were also unremarkable.
Figure 1.Axial non-contrast CT image showing a crescent shaped isodense subdural hematoma in the left hemisphere of the parietal lobe. The right hemisphere, ventricular system, and deep structures of the brain appear normal with no midline shift.

Although there are various effective modalities of treatment for CSDH, using burr-hole evacuation or Middle Meningeal Artery (MMA) embolization, there were no neurosurgery facilities or trained neurosurgeons to perform any invasive interventions.
Our patient's fluids were maintained with 500CC of Ringer's lactate solution and treatment was conservative with 200 mg/kg qd IV infused mannitol and 12 mg of IV infused dexamethasone over of 30 to 60 minutes to reduce intracranial pressure associated with brain edema. 50 mg qd captopril and 25 mg BID of atenolol were prescribed for control of hypertension. The patient was then transferred to the largest referral trauma center in Belize City, Belize, Karl Heusner Memorial Hospital (KHMH) for further expert intervention.
Though trauma still stands as the primary mechanism of CSDH it is often overlooked in the elderly population and consequently creates a degree of retrospective haze around its true time and place. 7 The most frequently presented symptoms in CSDH have been intellectual deterioration and change in personality which eerily mirror the presenting symptoms of mid-to-late stage AD10 Late-onset AD is most commonly diagnosed in patients after the age of 60 with a global prevalence of 6%.1,8
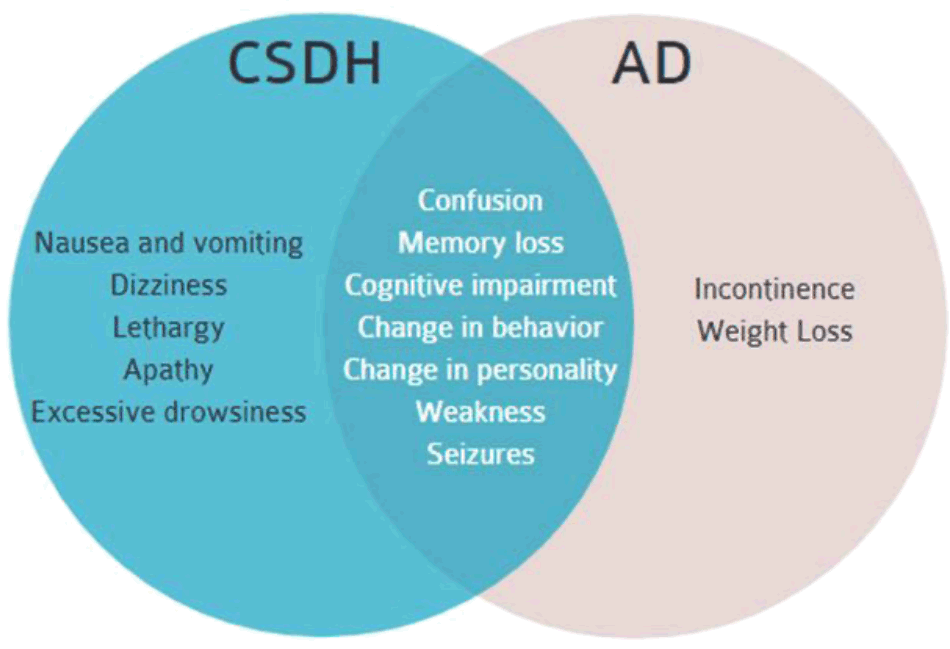
Symptoms of mid-to-late stage AD include confusion, cognitive impairment, change in behavior and personality, memory loss, weakness, seizures, weight loss and incontinence.11,12 Coincidentally, the initial presentation of a symptomatic CSDH may also include confusion, cognitive impairment, change in behavior and personality, memory loss, weakness and seizures.9 The use of antiplatelet and anticoagulants are also common risk factors for CSDH in the elderly population.
Figure 2.Symptoms of mid-to-late stage AD and CSDH. There is significant overlap which includes confusion, memory loss, cognitive impairment, change in behavior, and change in personality, weakness and seizures.2-6
We report this peculiar case because the initial presentation of recurrent subdural hematoma is likely to be masked by the symptoms of mid-to-late stage AD. While CSDH is treated most effectively by MMA embolization via an endovascular approach or by burr-hole evacuation, Belize, like many other developing countries currently lack the facilities and trained specialist to perform interventional neuroradiology procedures. Many of our health centers also lack neurosurgeons trained to perform the optimal lifesaving procedures.9
The morbidity and mortality rate in CSDH varies significantly worldwide.1 The overall in-hospital mortality index during admission is estimated at 15.6%.1 Overall outcome is good in patients who receive early surgical intervention with a morbidity and mortality rate about 16% and 6.5% respectively.1 However, the morbidity, mortality, and overall prognosis in patients with comorbid AD and CSDH requires further investigation.
In conclusion, it is important for physicians to recognize signs of early recurrent CSDH in the elderly and develop protocols to effectively distinguish the condition from that of mid-to-late stage AD. Especially since both conditions are comorbid. Of significance, CSDH ultimately leads to death, and therefore must not be confused with AD, especially in patients with a past cranial surgical history. Early management is a key factor in minimizing serious complications such as recurrence, seizures, and tension pneumocephalus.1,8 Despite the lack of adequate neurosurgical intervention, our patient was treated conservatively and stabilized with corticosteroids, beta blockers, angiotensin-converting enzyme (ACE) inhibitors and diuretic i.e. mannitol. Our patient was then transferred to the better equipped referral trauma center in Belize City, KHMH, for further expert intervention.
The author thanks Taiwan ICDF and l-Shou University School of Medicine for International Students, Taiwan, for the opportunity to continue developing as physician scientists. The authors also thank Paul Morgan Sr. for editing the manuscript.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Project Administration: PMM.
Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002 Feb;78(916):71-5.
Bature F, Guinn B-A, Pang D, Pappas Y. Signs and symptoms preceding the diagnosis of Alzheimer's disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open. 2017 Aug 28;7(8):e015746.
Prystupa A, Kurys-Denis E, topaty ski T, Baraniak J, Krupski W, Nogalski A, et al. Chronic subdural haematoma in a patient with arterial hypertension and Alzheimer's disease. J Pre Clin Clin Res. 2009;3(2):127-9.
Mijajlović MD, Pavlović A, Brainin M, Heiss W-D, Quinn TJ, Ihle-Hansen HB, et al. Post-stroke dementia - a comprehensive review. BMC Med. 2017 Jan I8;15(i):11..
Kuwahara S, Kawada M, Uga S. Chronic subdural hematoma with vasogenic edema in the cerebral hemisphere-case report. Neurol Med Chir (Tokyo). 2001 Apr;41 (4):196-200.
Sahyouni R, Tran D, Chen J. The effects of subdural hematoma on dementia, and a posible pathophysiological mechanism via blockage of dural lymphatics. Alzheimers Dement. 2016 July 24:12(7):P562.
Edlmann E, Giorgi-Coll S, Whitfield P, L. H. Carpenter K, J. Hutchinson P. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017 May 30;14(1):108.
Uno M, Toi H, Hirai S. Chronic Subdural Hematoma in Elderly Patients: Is This Disease Benign? Neurologia medico-chirurgica. Neurol Med Chir (Tokyo). 2017 Aug l5;57(8):402-9.
Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 2016 0ct-Dec;11(4):33O-42.
Duong S, Patel T, Chang F. Dementia: What pharmacists need to know. Can Pharm J (Ott). 2017 Feb 7;150(2):118-129.
Li X-L, Hu N, Tan M-S, Yu J-T, Tan L. Behavioral and psychological symptoms in Alzheimer's disease. BiOMed research international. 2014; 2014: 927804.
Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009 Feb 5;338:b158.
Paul Marcel Morgan, 1 School of Medicine for International Students, I-Shou University, Kaohsiung, Taiwan
2 Department of Accident and Emergency, Western Regional Hospital, Belmopan City, Belize
About the Author: Paul Morgan is currently a final year medical student in the four-year Doctor of Medicine (M.D.) Program at l-Shou University School of Medicine for International Students. Paul is also an adjunct instructor of biochemistry at the University of Belize.
Correspondence: Paul Marcel Morgan. Address: No. 1 號, Section 1, Xuecheng Road, Dashu District, Kaohsiung City, Taiwan 840. Email: buddymorganfagmail.com
Cite as: Morgan MP. Recurrent Subacute Subdural Hematoma in a 67-Year-Old Female with Late Alzheimer's Disease: A Case Report. Int J Med Students. 2019 May-Aug;7(2):38-40.
Copyright © 2019 Paul Marcel Morgan
International Journal of Medical Students, VOLUME 7, NUMBER 2, August 2019