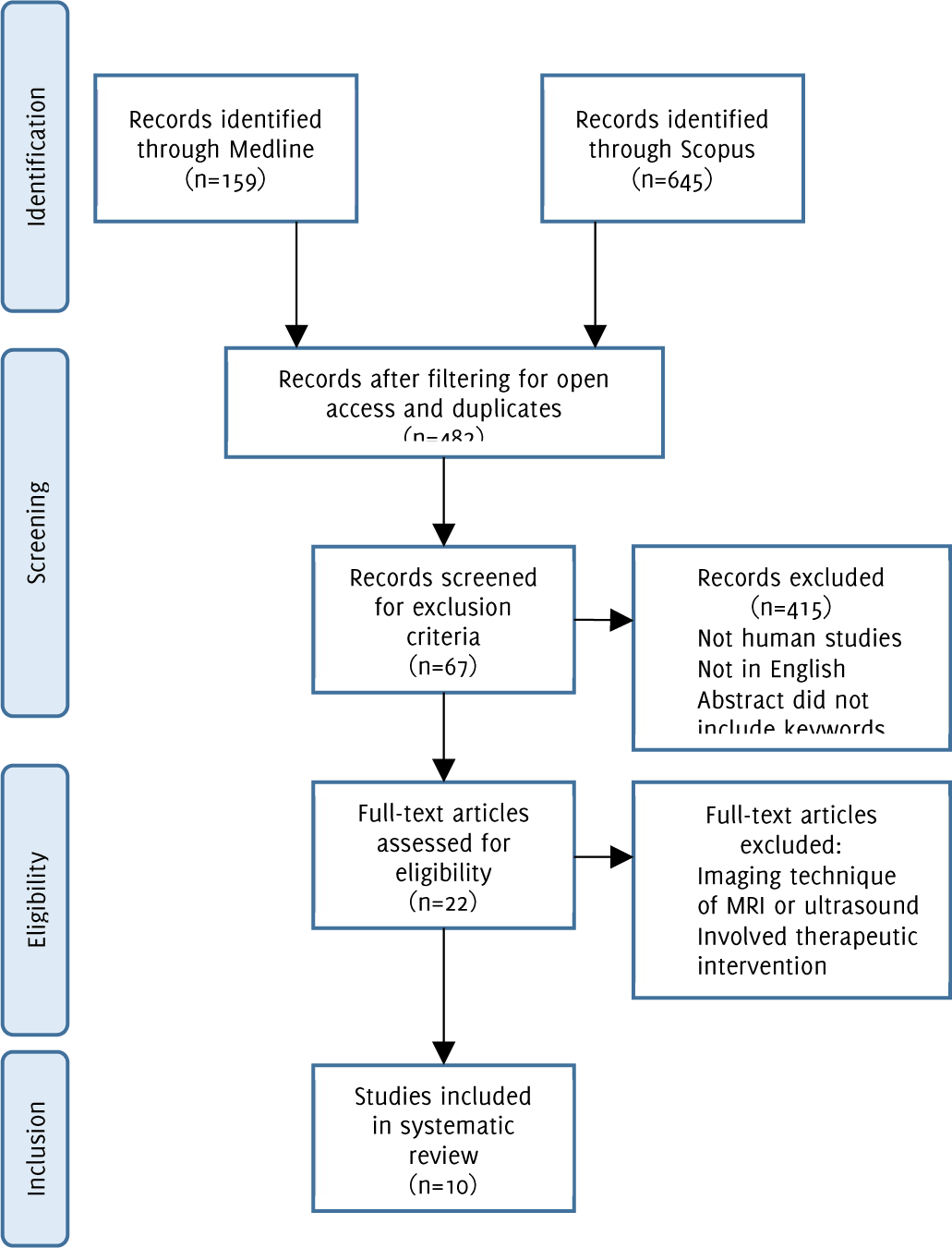
Flow diagram of study methodology with application of inclusion and exclusion criteria.
Shawn Stefan Albers1, Andrew Stanton Kucey1, Anish Engineer2
doi: http://dx.doi.org/10.5195/ijms.2019.380
Volume 7, Number 3: 82-91
Received 19 04 2019: Rev-recd 05 05 2019: Rev-recd 01 07 2019: Accepted 27 09 2019
ABSTRACT
Carotid artery disease (CAD) is associated with numerous risk factors, including hypertension, hyperlipidemia, hypercholesterolemia, diabetes mellitus, and smoking. In most patients, these systemic risk factors do not affect the carotid arteries equally, resulting in asymmetrical CAD. It is unclear if anatomic variations in the carotid arteries predispose an individual to formation of atherosclerotic CAD. Therefore, we wanted to assess (1) the inter-individual or intra-individual anatomical variations in the carotid arteries and (2) whether anatomical variations predispose the development of atherosclerotic CAD. We searched Medline and Scopus over the past 20 years as well as included article bibliographies. Two investigators independently screened abstracts and full-text articles; extracted data and assessed risk of bias. We included full-text primary articles that evaluated anatomical characteristics and the presence of CAD. A total of 8 articles were selected using the search parameters and an additional two articles were included after reviewing references of relevant papers. Evidence suggests that a low outflow/inflow ratio, elevated bifurcation height, and bifurcation angle are associated with increased risk for CAD. Additionally, tortuosity and kinking of the carotid arteries may affect the formation of CAD but coiling of the arteries which is a natural age-dependent process, does not affect CAD development. This review suggests there are anatomic variations in the carotid arteries that increase the risk of developing carotid artery disease. The most significant risk factors include a low outflow/inflow ratio, increased internal carotid artery tortuosity, elevated bifurcation height, and bifurcation angle.
Keywords: CT carotid angiogram; Carotid artery disease; Carotid bifurcation; Carotid artery anatomy; Carotid stenosis (Source: MeSH-NLM).
Carotid artery disease (CAD) is a vascular disease characterized by progressive narrowing of the blood vessel lumen due to atherosclerotic plaque deposition within the subendothelial lining.1 CAD is a leading cause of stroke, which is the third leading cause of mortality worldwide.2 Systemic risk factors such as hypertension, hyperlipidemia, diabetes mellitus, and smoking contribute to the formation of atherosclerotic plaques.3 Local factors such as hemodynamics and shear stress also influence plaque formation, thus displaying the multi-faceted pathogenesis of CAD.4 Atherosclerosis is regarded as a systemic disease, however, there is significant intraindividual variation in the extent to which the carotid arteries are affected.5,6 This suggests that there may be intraindividual features that predispose a particular artery to develop CAD.7
Blood vessel anatomy and geometry have a marked effect on both the initial formation of atherosclerotic plaques and the development of CAD.8 Atherosclerotic plaques preferentially deposit around the carotid bifurcation,9 disrupting blood flow in all directions and thereby contributing to the pathogenesis of CAD.10 A reduced outflow/inflow ratio, which compares the external carotid artery (ECA) and internal carotid artery (ICA) diameters to the common carotid artery (CCA), is an important indicator of plaque formation. A lower ratio can lead to reduced wall shear stress and an increased risk of endothelial damage, which would precipitate atherosclerotic CAD.11 It has been previously shown that the optimal ratio is 1.15; deviation from this can increase the risk of endothelial damage leading to atherosclerotic plaque formation.12 Initial atherosclerotic lesions occur early in fetal life, but do not have significant effects during childhood.13 Formation of these lesions depends on factors such as maternal hypercholesterolemia, susceptibility of the arteries, and numerous genetic factors.13 Aging coincides with marked increases in stress and anatomical changes in the carotid arteries.14 Increases in vessel diameter and tortuosity of the carotid arteries have been associated with normal aging and disease progression.14 Age-related degradation and fragmentation of the stabilizing elastin protein plays a role in the structural alterations seen in the carotid arteies.15 Interestingly, there is inter-ethnic variation of atherosclerotic plaque development at the carotid bifurcation, with blacks displaying a lower prevalence compared to Caucasians and Hispanics. This remains consistent in populations of blacks with an elevated vascular disease risk profile.
Although there are many known risk factors for the development of atherosclerotic CAD, both locally and systemically, many of these fail to address the presence of asymmetrical CAD within the same individual. This knowledge gap is important as it limits potential therapeutic interventions that would prevent CAD in certain populations. A comprehensive explanation for the presence of asymmetrical CAD is therefore needed to better understand the development and pathological progression of this disease. The aim of this review is to answer 2 key questions: Are there inter-individual or intra-individual anatomical variations in the carotid arteries? Do carotid artery anatomical variations predispose individuals to the development of atherosclerotic CAD?
An electronic search was conducted on Scopus and Medline (PubMed) to identify relevant publications investigating anatomical factors that may contribute to CAD. The following search parameters were used: “(Carotid artery diseases) AND (diagnostic imaging OR cerebral angiography) AND (anatomy OR anatomic) AND bifurcation”. Only studies involving humans, written in English and published in the past 20 years were considered for inclusion. The initial search resulted in 645 journal articles. By selecting articles published in the last 20 years, another 215 articles were excluded. Another 29 and 16 journals were eliminated by filtering out non-English and non-human studies, respectively. This yielded 385 articles for further screening (Figure 1). Using identical parameters as mentioned previously, a second search was conducted on Medline which yielded 159 articles. Filtering for articles published over 20 years ago eliminated 22 results. Another 3 and 24 articles were removed by including only journals published in English and human studies respectively. The total number of articles collected on Medline was 110 (Figure 1).
Figure 1Flow diagram of study methodology with application of inclusion and exclusion criteria.

The 385 Scopus and 110 Medline articles were combined on EndNote and 13 duplicates were removed. Using the exclusion criteria listed below, 482 articles were reduced to 67. These abstracts were independently reviewed by two investigators (SA, ASK) the titles and abstracts for imaging technique, therapeutic intervention, and diagnostic imaging. In total, 22 full articles were carefully reviewed by two investigators (SA, ASK) and 8 articles were included in the study from Scopus and Medline searches. Any discrepancies between the two investigators were resolved using a third investigator (AE). Additionally, the authors reviewed the bibliographies of the relevant articles included in this manuscript. An additional two articles were reviewed and selected for inclusion (Figure 1).
Studies were excluded based on the following parameters: abstracts not containing the word “carotid”; abstracts not containing the words “anatomy or anatomical or geometry; articles using MRI or Ultrasound as their main imaging modality; articles that investigated a therapeutic intervention. Inclusion parameters included: articles studying the anatomy or geometry of the carotid bifurcation; articles studying the anatomy or geometry of the internal carotid artery, external carotid artery and/or common carotid artery; and articles using Computed Tomography (CT) scans.
The following information was extracted from each article: Objectives; population demographic including mean age and range, and sex; sample size; methods and selection criteria; key findings; results; strengths and limitations.
Five studies, summarized in Table 1, focused on inter-individual and intra-individual anatomical variations at the carotid bifurcation in patients with CAD. The outflow/inflow ratio ranged from 0.38 to 1.28 between individuals, while 42% of patients with unilateral CAD had greater than 25% side-to-side difference in outflow/inflow ratio (P<0.0001).17 There was a positive linear relationship between the ICA angle and degree of ICA stenosis (OR, 1.05 per degree increment).17 An ICA angle of greater than 31.5o correlated with greater ICA stenosis.18 Another study showed a positive correlation between bifurcation angle and bifurcation height, with a 3.34o increase in the angle for each 1/3 vertebral body elevation of the origin of the carotid bifurcation (P<0.01).19 Contradictory evidence from Kemenskiy et al., showed a bifurcation angle of 25.36o 9.16 in CAD and 47.77o 25.61 in non-CAD patients (P=0.01).20
Table 1Anatomic risk factors for the development of CAD.
| Author, Date, Location, Title | Objectives | Type ofstudy, Sample size | Mean Age (Range) | Sex, % | Methods, Selection criteria | Key findings | Strengths/Limitations |
|---|---|---|---|---|---|---|---|
| Schulz U.G.R. and Rothwell P.M. (2001) UK17 Major Variation in Carotid Bifurcation Anatomy A Possible Risk Factor for Plaque Development | Assess the extent of variation of the carotid bifurcation between and within individuals | Retrospective Cohort StudySample size = 3018 | Unknown | Unknown | Measured arterial diameters of the ICA, ECA, CCA and bulb and calculated ratios from
CT angiograms Inclusion criteria: <30% ICA or CCA stenosis Exclusion criteria: >30% ICA or CCA stenosis |
Large variation between individuals: ECA range between 0.5 to 1.3x size of ICA. Outflow area range from 62% less to 28% more than Inflow area Intra-individual variation: 42% of people had >25% asymmetry in outflow/inflow between left and right carotids. |
Strengths: Large population from multiple centers around Europe Clear inclusion exclusion criteria Use of ratios allowed for consistent analysis of different CT angiograms Limitations: Biased population of predominately elderly with established vascular disease. Single observer with a Jeweler's eyepiece, not computerized. Different CT angiogram quality and technique within database |
| Phan T.G., et al (2012) Australia18 Carotid Artery Anatomy and Geometry as Risk Factors for Carotid Atherosclerotic Disease |
Assess the relationship between carotid artery anatomy and geometry and ICA stenosis | Case-control Study Sample size = 178 |
68.4 (unknown) | M, 65% | Bifurcation and vessel angles and vessel radii were measured from 3D reconstructed
segmented CT angiograms. Inclusion criteria: Patients with established carotid artery disease. Exclusion criteria: Unknown |
Positive linear relationship between ICA angle and degree of ICA stenosis. Increase in angle showed increased ICA stenosis. ICA angle >31.3° correlated with ICA stenosis. ICA radius was an independent predictor of ICA stenosis. |
Strengths: Large sample size of patients with established carotid artery disease. Measurement protocol had multiple controls to limit variability. 3D reconstruction software used for consistency. Limitations: Selection bias of patients with high vascular risks. |
| Kamenskiy A.V., at al (2015) Nebraska20 Age and disease-related geometric and structural remodeling of the carotid artery |
Assess if age-related carotid artery geometry changes affect the development of atherosclerotic carotid artery disease | Prospective Cohort Study Sample size = 32 |
Healthy 43 (15-64) Unilateral CAD 68 (49-86) |
Healthy M, 40% Unilateral CAD M, 70% |
Carotid artery diameter, tortuosity and bifurcation angle were measured in 3D reconstructed
CT angiograms. Inclusion criteria: Patients with unilateral carotid artery disease. Exclusion criteria: Patients with bilateral carotid artery disease |
For every decade of life increases in bulb diameter (0.64mm), bifurcation angle (10°)
and tortuosity of the ICA and CCA. Larger bulb diameter, smaller bifurcation angle, increased tortuosity of CCA, and reduced tortuosity of ICA are seen in CAD vs non-diseased. Geometrical changes correlated with degradation and fragmentation of intramural elastin. |
Strengths: Correlated findings with histological elastin-staining to assess structural changes.
3D reconstruction and computerized measurements increased accuracy. Limitations: Type 1 error due to low sample size. Did not follow patients over time (longitudinal study to assess effect of aging). |
| De Syo S., Franjic B.D., Lovricevic I., Vukelic M. and Palenkic H. (2004) Croatia19 Carotid Bifurcation and Position and Branching Angle in Patients with Atherosclerotic Carotid Disease |
Assess the correlation between carotid bifurcation height and angle in the neck in carotid artery disease patients | Cross-sectional Study Sample size = 154 | Male 57.2 (24-76) Female 58.4 (27-79) |
M, 75% | 154 bi-plane orthogonal aortic arch arteriograms were obtained from symptomatic carotid
artery disease patients and bifurcation height in relation to cervical spine and bifurcation
angle were measured. Inclusion criteria: Symptomatic carotid artery disease patient. Exclusion criteria: fully Occluded bifurcation and poor imaging |
Positive correlation between bifurcation height and branching angle. The bifurcation angle increases 3.34° for each 1/3 vertebral body elevation of bifurcation height |
Strengths: Used a standardized and accepted method of measuring bifurcation height. Large range of patient ages (24-79). Limitations Statistical analysis was not included in methods. Outdated, noncomputerized method of bifurcation angle measurement. Confounding variables such as degree of carotid artery disease on anatomy and geometry not taken into consideration. |
| Cappabianca S., Somma F., Negro A., Rotondo M., Scuotto A. and Rotondo A. (2016) Italy21 Extracranial internal carotid artery: anatomical variations in asymptomatic patients |
Assess anatomical variations in the ICA and estimate the prevalence within the sample population | Prospective Cohort Study Sample size = 316 |
64 (37-81) | M, 54% | ICA anatomy and deformities were assessed using CT angiography and MRI. Inclusion criteria: All patients with angiograms performed. Exclusion criteria: Patients with metal devices. Equivocal interpretation. |
CT angiograms detected 100% of ICA abnormalities, MRI detected 89.9%. Kinking/coiling of ICA was present in 20.7% of patients. Kinking in patients > 55 years old. Coiling in patients < 37 years old. | Strengths: All images independently evaluated by radiologist. Compared accuracy of MRI to CT
angiogram. Limitations: Area of further research not stated Presence of carotid artery disease not reported Effect of age on anatomical variation not discussed |
25% of patients with atherosclerotic CAD had a positive correlation between kinking of the ICA and high bifurcation height, whereas only 3.2% of patients showed ICA kinking with medium and low bifurcation height (P<0.01).19 ICA kinking and coiling was present in 20% of patients with CAD, with 80% presenting bilaterally and 20% unilaterally. Kinking was associated with aging, and patients greater than 55 years old have been shown to be at an elevated risk of this anatomical variation.21
Four studies summarized in Table 2 investigated the demographic differences in carotid anatomy in both healthy and CAD patients. ICA stenosis was independently associated with age (OR, 1.05 per year increment), male sex (OR, 1.72) and current or past smoking history (OR, 1.85).18 Males were more likely to have a point of maximal stenosis in the ICA (OR, 2.29, P=0.001), however, women were more likely to have ECA stenosis (OR, 1.54, P<0.0001) and a higher outflow/inflow ratio (0.77 F, 0.71 M, P<0.001).22
Table 2Demographic variations in carotid artery anatomy in patients with and with CAD.
| Author, Date, Location, Title | Objectives | Type of study, Sample size | Mean Age (Range) | Sex, % | Methods, Selection criteria | Key findings | Strengths/Limitations |
|---|---|---|---|---|---|---|---|
| Schulz U.G.R. and Rothwell P.M. (2001) UK22 Sex Differences in Carotid Bifurcation Anatomy and the Distribution of Atherosclerotic Plaque |
Assess any anatomical variation at the carotid bifurcation between sexes | Retrospective Cohort Study Sample size = 3018 |
Male 62.1 (unknown) Female 62.3 (unknown) |
M, 72% | Vessel diameters at disease free areas were measured on CT angiograms and ICA/CCA,
ECA/CCA, ICA/ECA, bulb/CCA and outflow/inflow ratios were calculated. Inclusion criteria: Angiograms with <50% stenosis. Contralateral vessels with no disease. Exclusion criteria: Angiograms with >50% stenosis. |
Average ICA/CCA, ICA/ECA and outflow/inflow were larger in women vs men. Lower average
outflow/inflow ratio in men. Women showed more stenosis in ECA, men had more stenosis distal to carotid bulb. |
Strengths: Large population from multiple centers around Europe. Ratios eliminate magnification difference in CT angiograms. Exclusion criteria eliminated much of the effect of atherosclerotic disease on anatomy. Limitations: Uneven populations (2168 male, 850 female). CT angiograms from different centers with different techniques and skills. Anatomical study should only involve non-atheromatous individuals. Single observer with Jeweler's eyepiece for measurement. |
| Sehiril U.S., Yalin A., Tulay C.M., Cakmak Y.O. and Gurdal E. (2005) Turkey23 The diameters of common carotidartery and its branches in newborns |
Assess the average diameters of the CCA, ICA, ECA and outflow/inflow ratio in newborns | Cross-sectional Study Sample size = 20 |
Newborns (Gestational week 34-40) | M, 55% | Fixed carotid arteries were dissected from newborn cadavers and vessel diameters were
measured. Inclusion criteria: Available cadavers. Exclusion criteria: Unknown. |
CCA, ECA, ICA diameter larger in males. CCA, ECA, ICA diameter and outflow/inflow greater on the right vs the left in both sexes. Outflow/inflow ratio larger in males. | Strengths: Rare population. Simple and accurate measurement. Limitations: Small sample size. No significant difference. No exclusion criteria. Unknown ethical approval. |
| McNamara J.R., Fulton G.J. and Manning B.J. (2015) Ireland24 Three-dimensional Computed Tomographic Reconstruction of the Carotid Artery: Identifying High Bifurcation |
To define a reproducible method for identifying patients with high carotid bifurcations | Retrospective Cross-sectional Study Sample size = 86 |
68 (20-90) | M, 54% | 3D reconstructed CT angiograms were used to assess the curved length and straight-line
distance of the ICA. Bifurcation height was measured relative to 8 anatomical landmarks. Inclusion criteria: Patients with symptomatic or asymptomatic carotid artery disease. Exclusion criteria: Occlusion of the ICA and abnormal positioning of patient. |
Measuring the distance of the bifurcation from the mastoid process gives the best
indication of a high bifurcation. Bifurcations within a distance 5cm of mastoid process is likely to be in the highest quartile (82.9% sensitive, 80.1% specific). No straight line distance difference between left and right ICA. |
Strengths: Population specific for those likely to receive a carotid endarterectomy. High level of accuracy using 3D reconstruction of thin slice CT angiography. Inter-observer accuracy of measurement 0.996. Limitations: Small population size. Current software cannot calculate straight line distance. No assessment of intra-operative clinical correlation for relevance of a high carotid bifurcation. |
| Koch S., Nelson D., Rundek T., Mandrekar J. and Rabinstein A. (2009) Florida25 Race-ethnic Variation in Carotid Bifurcation Geometry |
Assess structural differences in carotid bifurcation anatomy between Caucasians, African Americans, and Caribbean Hispanics | Retrospective Cohort Study Sample size = 153 |
59.8 (unknown) | M, 54.4% | CT angiograms from 3 different races were analyzed and the CCA, ICA, ECA and carotid
bulb diameters were measured. Inclusion criteria: <50% vessel stenosis. Exclusion criteria: >50% vessel stenosis based on the NASCET criteria. |
African Americans had lower ICA/CCA and ICA/ECA but an elevated ECA/CCA ratio compared to Caucasians and Hispanics. There were no differences between Caucasians and Hispanics. There were no differences in cross-sectional outflow/inflow ratio between the 3 groups. | Strengths: Observer was blinded to ethnic group. 2 observers used for measurements (high inter-observer accuracy 0.96). Limitations: Hospital-based patient cohort, not representative of global population. Variation in ratios is small but statistically significant (may not be physiologically significant). Age variation between groups which may contribute to anatomical variation. |
Neonates did not have marked differences in outflow/inflow ratios or carotid artery diameter when comparing males and females.23 For every decade of life increase there were concurrent increases in: carotid bulb diameter (0.64mm), ICA tortuosity (0.04), CCA tortuosity (0.03) and bifurcation angle of 10o (p<0.05).20 These geometrical changes correlated with degradation and fragmentation of intramural elastin.20 Tortuosity was most accurately measured using 3D reconstructed CT angiograms, using a computer generated curved length (CL) with a multi-planar measured and calculated straight-length diameter (SLD).24 African Americans had a lower ICA/CCA ratio (P<0.01) compared to Caucasians and Hispanics, however there was no significant difference in outflow/inflow ratio between the three race-ethnic groups (P>0.05).25
The final study, summarized in Table 3, investigated the association between carotid bifurcation and pathogenesis of CAD. There was no significant difference between the outflow/inflow ratio between the asymptomatic (0.72) and symptomatic (0.71) sides (p=0.95). Furthermore, there was no association between bifurcation anatomy and plaque ulceration, with an outflow/inflow ratio of 0.69 in ulcerated plaques and 0.72 in non-ulcerated plaques (p=0.06).26
Table 3Carotid artery anatomy and the pathogenesis of CAD.
| Author, Date, Location, Title | Objectives | Type of study, Sample size | Mean Age (Range) | Sex, % | Methods, Selection criteria | Key findings | Strengths/Limitations |
|---|---|---|---|---|---|---|---|
| Schulz U.G.R. and Rothwell P.M (2003) UK26 Association between Arterial Bifurcation Anatomy and Angiographic Plaque Ulceration among 4,627 Carotid Stenoses |
Assess the relationship between carotid artery vessel anatomy and plaque stability in a human model | Retrospective Cohort Study Sample size = 3018 |
Unknown | Unknown | CT Angiograms were studied for carotid artery anatomy and plaque ulceration in randomized
patients from the European Carotid Surgery Trial. Inclusion criteria: Presence of symptomatic carotid artery disease. Exclusion criteria: Poor imaging, near full occlusion and contralateral carotid bifurcation with no atheromatous plaque evidence. |
No association between bifurcation anatomy and plaque ulceration in affected artery. High ECA/CAA and outflow/inflow ratio show reduced plaque ulceration but not significant. | Strengths: Large population from multiple centers across Europe. Data was analyzed by two independent observers. Limitations: Measurements made by Jeweler's eyepiece, not computerized. Inter-observer agreement on measurement was 0.79. Biased cohort selected from the European Carotid Surgery Trial, not representative of the general population. |
Each of the 10 selected journal articles were critically appraised using the EBL criteria, with the results summarized in Table 4. The studies had overall validity scores that ranged from 78.2 to 88.0% (Appendix 1). The numerical and statistical values of each study are summarized in Appendix 2.
Table 4Critical appraisal and selection score of reviewed articles based on the EBL critical appraisal checklist.
| Article | Population Validity Score (%) | Data Collectio n Validity Score (%) | Study Design Validity Score (%) | Results Validity Score (%) | Overall Validity Score (%) |
|---|---|---|---|---|---|
| McNamara et al (2015) | 60.0 | 100 | 100 | 83.3 | 86.6 |
| Schulz et al (2001) | 60.0 | 100 | 100 | 83.3 | 86.9 |
| De Syo et al (2005) | 80.0 | 57.1 | 80 | 100 | 78.2 |
| Koch et al (2009) | 75.0 | 100 | 80.0 | 83.3 | 84.6 |
| Schulz et al (2001) | 60.0 | 85.7 | 100 | 83.3 | 82.6 |
| Schulz et al (2003) | 60.0 | 100 | 100 | 83.3 | 86.9 |
| Phan et al (2012) | 50.0 | 100 | 100 | 83.3 | 83.3 |
| Kamenskiy et al (2015) | 57.1 | 100 | 100 | 100 | 88.0 |
| Sehirli et al (2005) | 60.0 | 85.7 | 80.0 | 83.3 | 78.3 |
| Cappabianca et al (2016) | 100 | 85.7 | 100 | 66.7 | 88.0 |
This review summarizes inter-individual and intra-individual carotid artery bifurcation variations seen in patients with CAD. It also highlights anatomical and demographic factors that are associated with CAD pathogenesis. Finally, it provides a better understanding of why people develop unilateral CAD when both sides are equally exposed to systemic risk factors.
A reduced outflow/inflow ratio is a significant predictor of the development of atherosclerotic CAD. A lower ratio was found in patients with unilateral CAD,17 in males22 and in association with increased plaque ulceration.26 The stability of atherosclerotic plaques is directly influenced by local hemodynamic and mechanical forces.26 Mechanical forces arise during the cardiac cycle, whereby pressure changes lead to alternating compression and tension on a plaque.26 A reduction in the outflow/inflow ratio can change local hemodynamic forces, resulting in an impaired and reduced flow energy. This can increase local stress on the vasculature, and lead to endothelial damage and plaque formation.11 Surprisingly, blacks showed no difference in outflow/inflow ratio despite significantly different ICA, ECA, and CCA dimensions compared to Caucasians and Hispanics.25 Blacks are regarded to have a higher adverse vascular risk profile but a lower prevalence of atherosclerotic CAD. Carotid anatomy and geometry may still play a role in this disparity, however further investigations are required.
Carotid artery geometry and anatomy change with physiological aging. At birth, male and female carotid anatomies are very similar, with outflow/inflow ratios close to the predicted optimal value of 1.15.23 This optimal outflow/inflow ratio has been well established for decades, and any deviation from this can lead to greater local stress and endothelial damage.12 A reduced outflow diameter can cause an increased pulse wave pressure exerted on the surrounding endothelial lining of the blood vessel, which can lead to damage and plaque development.23 Unsurprisingly, elderly patients with established CAD demonstrate significant deviation from the optimal outflow/inflow ratio, averaging as low as 0.67.26 Increases in ICA kinking,21 carotid bulb diameter, ICA and CCA tortuosity, and bifurcation angle are more prevalent as the population ages.20 These alterations in the absence of disease are correlated with degradation and fragmentation of intramural elastin.20 Elastin provides the retractive force, which counteracts traction and pressure forces, thereby stabilizing the artery and maintaining its integrity and straight shape.15 Secondly, there are marked differences in elastin orientation within the ICA and CCA. Elastin in the CCA is found in both the circumferential and longitudinal directions; in the ICA it is predominately found longitudinally within the muscular layer.20 Degeneration of elastin in the longitudinal direction likely results in increased tortuosity in both the ICA and CCA.20 There are differences in tortuosity between the CCA and ICA in patients with atherosclerotic CAD. Straighter ICAs and more tortuous CCAs are present in CAD, which appears to be linked to plaque deposition within the ICA.20 Furthermore, ICA kinking may be a predisposing factor to the development of atherosclerotic plaques and can be unilateral or bilateral.21 The threshold at which geometric and anatomic changes may precipitate or protect from the formation of atherosclerotic CAD is not currently known. The invasive nature of CT angiography makes it unethical to subject healthy individuals to this procedure. Therefore, these areas of study require further investigation.
There is controversy in the literature concerning the bifurcation angle and the presence of CAD. A significant association between elevated bifurcation angle of the ICA and the presence of CAD within the ICA was seen in a study by Phan et al (OR).18 This finding was based on a large retrospective cohort study of 178 patients. Kamenskiy et al., on the other hand, found a more acute bifurcation angle to be associated with greater CAD.20 These findings were based on a smaller sample size and examined drastically different populations, which included older patients with less severe CAD. Phan et al. focused their study on patients with advanced stenosis and larger ICA bulbs, which have been shown to laterally displace the arterial centerline causing an increase in bifurcation angle.18 Simulation studies showed elevated bifurcation angles were associated with reduced wall shear stress, precipitation of the formation of fatty streaks, and creation of atherosclerotic plaques.18 These results were taken one step further to show an association between the bifurcation angle and bifurcation height in CAD patients by De Syo et al.19 A positive correlation between bifurcation height and bifurcation angle was seen in patients with unilateral CAD.19 It is unknown whether bifurcation angle and height are independent or synergistic risk factors for the development of CAD.
This study summarizes 10 critically appraised journal articles that investigated the effect of carotid anatomy on the presence of CAD. These studies were conducted all around the world, most using large sample sizes, thereby giving a global perspective on anatomical variations in CAD. This study compiles results from studies investigating the link between carotid anatomy and CAD, an area where there is currently a paucity of data available.
The current study was limited to articles published in English, which exclude possible relevant manuscripts on this topic. Despite using similar imaging techniques, the articles reviewed had significantly different measurement techniques. This discrepancy may account for some inter-study variability. Many of the reviewed articles studied specific populations, leading to high selection bias. Finally, a literature review is designed to minimize researcher bias, however, this is not completely avoidable due to the requirement for individual judgment on which results to include in the study
This is a literature review which highlights the significant association between carotid artery anatomy and geometry in initiation and progression of CAD. Carotid artery anatomy is optimal and equal bilaterally at birth, but changes with age. These changes appear to predispose an individual to the development of atherosclerotic CAD and add to an already extensive list of risk factors. Despite the extensive literature available highlighting this, there is need for further research in order to understand the exact pathogenesis of CAD. A longitudinal study, following specific cohorts over time, will give the best indication on natural and pathogenic changes of carotid anatomy and the development of CAD.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: SA. Methodology: SA, ASK, and AE. Formal Analysis: SA. Investigation: SA, ASK, and AE. Writing – Original Draft: SA. Writing – Review & Editing: SA, ASK, and AE. Visualization: SA, ASK, AE. Supervision: SA. Project Administration: SA.
Appendix 1 EBL critical appraisal checklist.| EBL Critical Appraisal Checklist | Schulz et al (2003) | Schulz et al (2001) | Phan et al (2012) | Kamenskiy et al (2015) | Koch et al (2009) | De Syo et al (2004) | McNamara et al (2015) | Schulz et al (2001) | Sehirli et al (2005) | Cappabianca et al (2016) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Section A: Population | Is the study population representative of all users, actual and eligible, who might be included in the study? | N | N | N | Y | N | Y | N | N | Y | Y |
| Are inclusion and exclusion criteria definitively outlined? | Y | Y | N | Y | Y | Y | Y | Y | N | Y | |
| Is the sample size large enough for sufficiently precise estimates? | Y | Y | Y | N | Y | Y | Y | Y | N | Y | |
| Is the response rate large enough for sufficiently precise estimates? | N/A | N/A | Y | N/A | Y | N/A | N/A | N/A | N/A | N/A | |
| Is the choice of population bias free? | N | N | N | N | Y | Y | N | N | Y | Y | |
| Were participants randomized into groups? | N/A | N/A | N/A | N | N | N/A | N/A | N/A | N/A | Y | |
| Were the groups comparable at baseline? | N/A | N/A | N/A | Y | Y | N/A | N/A | N/A | N/A | Y | |
| If groups were not comparable at baseline, was incomparability addressed by the authors in the analysis? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Was informed consent obtained? | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | |
| Section A Total | 60.00 | 60.00 | 50.00 | 57.10 | 75.00 | 80.00 | 60.00 | 60.00 | 60.00 | 100.00 | |
| Section B: Data Collection | Are data collection methods clearly described? | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| If a face to face survey, were inter-observer and intra-observer bias reduced? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Is the data collection instrument validated? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| If based on regularly collected statistics, are the statistics free from subjectivity? | Y | Y | Y | Y | Y | U | Y | Y | Y | U | |
| Does the study measure the outcome at a time appropriate for capturing the intervention's effect? | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is the instrument included in the publication? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Are questions posed clearly enough to be able to elicit a precise answer? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Were those involved in data collection not involved in delivering a service to the target population? | Y | Y | Y | Y | Y | N | Y | Y | N | Y | |
| Section B Total | 100.00 | 85.70 | 100.00 | 100.00 | 100.00 | 57.10 | 100.00 | 100.00 | 85.70 | 85.70 | |
| Section C: Study Design | Is the study type/methodology utilized appropriate? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Is there face validity? | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | |
| Is the research methodology clearly stated at a level of detail that would allow its replication? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Was ethics approval obtained? | Y | Y | Y | Y | Y | U | Y | Y | U | Y | |
| Are the outcomes clearly stated and discussed in relation to the data collection? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Section C Total | 100.00 | 100.00 | 100.00 | 100.00 | 80.00 | 80.00 | 100.00 | 100.00 | 80.00 | 100.00 | |
| Section D: Results | Are all the results clearly outlined? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Are confounding variables accounted for? | Y | Y | Y | Y | Y | Y | N | Y | N | Y | |
| Do the conclusions accurately reflect the analysis? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Is subset analysis a minor, rather than a major, focus of the article? | N | N | N | Y | Y | Y | Y | U | Y | Y | |
| Are suggestions provided for further areas to research? | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | |
| Is there external validity? | Y | Y | Y | Y | U | Y | Y | Y | Y | U | |
| Section D Total | 83.30 | 83.30 | 83.30 | 100.00 | 83.30 | 100.00 | 83.30 | 83.30 | 83.30 | 66.70 | |
| Overall Validity | 86.9 | 82.6 | 83.3 | 88.0 | 84.6 | 78.2 | 86.6 | 86.9 | 78.3 | 88.0 | |
| Author, Date, Location | Values | Statistics | Results |
|---|---|---|---|
| Schulz U.G.R. and Rothwell P.M (2003) UK26 Association between Arterial Bifurcation Anatomy and Angiographic Plaque Ulceration among 4,627 Carotid Stenoses |
Outflow/inflow
|
P = 0.95 P = 0.06 |
Outflow/inflow ratio did not vary much between symptomatic and asymptomatic sides Outflow/inflow ratio did not significantly affect plaque ulceration on symptomatic side |
| Schulz U.G.R. and Rothwell P.M.(2001) UK17 Major Variation in Carotid Bifurcation Anatomy A Possible Risk Factor for Plaque Development |
Outflow/inflow asymmetry of > 25%
|
P < 0.05 | Large variation between individuals: ECA range between 0.5 to 1.3x size of ICA Outflow area range from 62% less to 28% more than Inflow area Intra-individual variation: 42% of people had >25% asymmetry in outflow/inflow between left and right carotids |
| Phan T.G., et al (2012) Australia18 Carotid Artery Anatomy and Geometry as Risk Factors for Carotid Atherosclerotic Disease |
Symmetrical stenosis
|
P < 0.05 | Positive linear relationship between ICA angle and degree of ICA stenosis – increase
in angle showed increased ICA stenosis ICA angle >31.3° correlated with ICA stenosis ICA radius was an independent predictor of ICA stenosis |
| Kamenskiy A.V., at al (2015) Nebraska20 Age and disease-related geometric and structural remodeling of the carotid artery |
Anatomical increases with each decade of life
|
P < 0.05 P = 0.01 |
Bifurcation angle and tortuosity of the ICA and CCA increases each decade of life Smaller bifurcation angle, increased tortuosity of CCA and reduced tortuosity of ICA are seen in CAD vs non-diseased Geometrical changes correlate with degradation and fragmentation of intramural elastin |
| Koch S., Nelson D., Rundek T., Mandrekar J. and Rabinstein A. (2009) Florida25 Race-ethnic Variation in Carotid Bifurcation Geometry |
Outflow/inflow ratio
|
P < 0.05 | African Americans had lower ICA/CCA and ICA/ECA but an elevated ECA/CCA ratio compared
to Caucasians and Hispanics There were no differences between Caucasians and Hispanics There were no differences in cross-sectional outflow/inflow ratio between the 3 groups |
| De Syo S., Franjic B.D., Lovricevic I., Vukelic M. and Palenkic H. (2004) Croatia19 Carotid Bifurcation and Position and Branching Angle in Patients with Atherosclerotic Carotid Disease |
Average bifurcation angle
|
P < 0.01 | Positive correlation between bifurcation height and branching angle The bifurcation angle increases 3.34° for each 1/3 vertebral body elevation of bifurcation height |
| McNamara J.R., Fulton G.J. and Manning B.J. (2015) Ireland24 Three-dimensional Computed Tomographic Reconstruction of the Carotid Artery: Identifying High Bifurcation |
Curved Length ICA
|
Measuring the distance of the bifurcation from the mastoid process gives the best
indication of a high bifurcation Bifurcations within a distance 5cm of mastoid process is likely to be in the highest quartile (82.9% sensitive, 80.1% specific) No straight line distance difference between left and right ICA |
|
| Schulz U.G.R. and Rothwell P.M. (2001) UK22 Sex Differences in Carotid Bifurcation Anatomy and the Distribution of Atherosclerotic Plaque |
ICA/CCA
|
P < 0.0001 P = 0.44 P < 0.0001 P < 0.001 P = 0.001 P < 0.0001 |
Average ICA/CCA, ICA/ECA and outflow/inflow were larger in women vs men Lower average outflow/inflow ratio in men Women showed more stenosis in ECA, men had more stenosis distal to carotid bulb |
| Sehiril U.S., Yalin A., Tulay C.M., Cakmak Y.O. and Gurdal E. (2005) Turkey23 The diameters of common carotid artery and its branches in newborns |
CCA
|
P < 0.05 | CCA, ECA, ICA diameter larger in males CCA, ECA, ICA diameter and outflow/inflow greater on the right vs the left in both sexes Outflow/inflow ratio larger in males |
| Cappabianca S., Somma F., Negro A., Rotondo M., Scuotto A. and Rotondo A. (2016) Italy21 Extracranial internal carotid artery: anatomical variations in asymptomatic patients |
Imaging vascular anomaly detection
|
P < 0.05 | CT angiograms detected 100% of ICA abnormalities, MRI detected 89.9% Kinking/coiling of ICA was present in 20.7% of patients Kinking in patients > 55 years old Coiling in patients < 37 years old |
1.Prasad K. Pathophysiology and Medical Treatment of Carotid Artery Stenosis. Int J Angiol. 2015 Sep;24 (3):158–72.
2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012Jan3;125 (1):188–97.
3.Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis. 2013 Apr;227 (2):193–200.
4.Richardson PD, Davies MJ, Born GV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989Oct21;2 (8669):941–4
5.Gnasso A, Irace C, Carallo C, De Franceschi MS, Motti C, Mattioli PL. In vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke. 1997 May;28 (5):993–8.
6.Ruan L, Chen W, Srinivasan SR, Sun M, Wang H, Toprak A. Correlates of common carotid artery lumen diameter in black and white younger adults: the Bogalusa Heart Study. Stroke. 2009 Mar;40 (3):702–7.
7.Goubergrits L, Affeld K, Fernandez-Britto J, Falcon L. Geometry of the human common carotid artery. A vessel cast study of 86 specimens. Pathol Res Pract. 2002;198(8):543–51.
8.Karino T, Goldsmith HL. Particle flow behavior in models of branching vessels. II. Effects of branching angle and diameter ratio on flow patterns. Biorheology. 1985;22(2):87–104.
9.Fabris F, Zanocchi M, Bo M, Fonte G, Poli L, Bergoglio I. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke. 1994 Jun;25 (6):1133–40.
10.Lee SW, Antiga L, Spence JD, Steinman DA. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke. 2008 Aug;39 (8):2341–7.
11.Spelde AG, de Vos RA, Hoogendam IJ, Heethaar RM. Pathological-anatomical study concerning the geometry and atherosclerosis of the carotid bifurcation. European journal of vascular surgery. Eur J Vasc Surg. 1990 Aug;4 (4):345–8.
12.Gosling RG, Newman DL, Bowden NL, Twinn KW. The area ration of normal aortic junctions. Aortic configuration and pulse-wave reflection. Br J Radiol. 1971 Nov;44 (527):850–3.
13.Napoli C, Witztum JL, de Nigris F, Palumbo G, D'Armiento FP, Palinski W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation. 1999Apr20;99 (15):2003–10.
14.Del Corso L, Moruzzo D, Conte B, Agelli M, Romanelli AM, Pastine F. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 1998 May;49 (5):361–71.
15.Zegers E, Meursing B, Zegers E, Oude Ophuis A. Coronary tortuosity: a long and winding road. Neth Heart J. 2007 May;15 (5):191–5.
16.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996 Nov;27 (11):1974–80.
17.Schulz UG, Rothwell PM. Major variation in carotid bifurcation anatomy: a possible risk factor for plaque development? Stroke. 2001 Nov;32 (11):2522–9.
18.Phan TG, Beare RJ, Jolley D, Das G, Ren M, Wong K. Carotid artery anatomy and geometry as risk factors for carotid atherosclerotic disease. Stroke. 2012 Jun;43 (6):1596–601.
19.De Syo D, Franjic BD, Lovricevic I, Vukelic M, Palenkic H. Carotid bifurcation position and branching angle in patients with atherosclerotic carotid disease. Coll Antropol. 2005 Dec;29 (2):627–32.
20.Kamenskiy AV, Pipinos, II, Carson JS, MacTaggart JN, Baxter BT. Age and disease-related geometric and structural remodeling of the carotid artery. J Vasc Surg. 2015 Dec;62 (6):1521–8.
21.Cappabianca S, Somma F, Negro A, Rotondo M, Scuotto A, Rotondo A. Extracranial internal carotid artery: anatomical variations in asymptomatic patients. Surg Radiol Anat. 2016 Oct;38 (8):893–902.
22.Schulz UG, Rothwell PM. Sex differences in carotid bifurcation anatomy and the distribution of atherosclerotic plaque. Stroke. 2001 Jul;32 (7):1525–31.
23.Sehirli US, Yalin A, Tulay CM, Cakmak YO, Gurdal E. The diameters of common carotid artery and its branches in newborns. Surg Radiol Anat. 2005 Nov;27 (4):292–6.
24.McNamara JR, Fulton GJ, Manning BJ. Three-dimensional computed tomographic reconstruction of the carotid artery: identifying high bifurcation. Eur J Vasc Endovasc Surg. 2015 Feb;49 (2):147–53.
25.Koch S, Nelson D, Rundek T, Mandrekar J, Rabinstein A. Race-ethnic variation in carotid bifurcation geometry. J Stroke Cerebrovasc Dis. 2009 Sep-Oct;18 (5):349–53.
26.Schulz UG, Rothwell PM. Association between arterial bifurcation anatomy and angiographic plaque ulceration among 4,627 carotid stenoses. Cerebrovasc Dis. 2003;15(4):244–51.
Shawn Stefan Albers, 1 BSc, MSc, University College Cork, School of Medicine, Ireland
Andrew Stanton Kucey, 1 BSc, MSc, University College Cork, School of Medicine, Ireland
Anish Engineer, 2 BMSc, PhD, Royal College of Surgeons in Ireland, School of Medicine, Dublin, Ireland
Thiago Henrique Roza, Editor
Mihnea-Alexandru Găman, Editor
Madeleine Jemima Cox, Student Editor
Correspondence: Shawn Stefan Albers, Address: Gaol Walk, University College, Cork, T12 YN60, Ireland. Email: salbers@uwo.ca
Cite as: Albers, SS, Kucey, AS, Engineer, A, The Role of Intraindividual Carotid Artery Variation in the Development of Atherosclerotic Carotid Artery Disease: A Literature Review. Int J Med Students. 2019 Sep-Dec;7(3):82-91.
Copyright © 2019 Shawn Stefan Albers, Andrew Stanton Kucey, Anish Engineer
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 7, NUMBER 3, December 2019