PRISMA Flow Diagram
Natalie Wietfeldt1, Andrew J. Boileau2
doi: http://dx.doi.org/10.5195/ijms.2020.437
Volume 8, Number 2: 126-134
Received 01 10 2019: Rev-request 21 10 2019: Rev-request 13 01 2020: Rev-recd 17 12 2019: Rev-recd 23 03 2020: Accepted 05 05 2020
ABSTRACT
FKBP5 gene variants may predict antidepressant treatment response in individuals with Major Depressive Disorder. PubMed and Web of Science were searched systematically for articles studying individuals who had received a diagnosis of Major Depressive Disorder (MDD) and were given antidepressant treatment. Inclusion criteria were studies that researched FKBP5 and its variants and focused on antidepressant treatment response. Previous studies support a potential underlying epigenetic mechanism, demethylation at FKBP5 polymorphisms (rs1360780, rs3800373, rs9470080, and rs4713916) after experiencing childhood trauma, leading to increased hypothalamic-pituitary-adrenal (HPA) axis sensitivity and a propensity for the development of MDD. These polymorphisms informed the review, but additional polymorphisms (rs9380514, rs352428) were also considered. Studies conducted prior to 2008, reviews, meta-analyses, editorials, and non-research-based articles were excluded. Studies examined in this article suggest FKBP5 polymorphism rs4713916 and FKBP5 RNA levels may be associated with antidepressant response. Variants rs1360780, rs3800373, and rs9470080 were associated with both positive response and non-response or lack of remission. Variants rs9380514, rs352428, and rs936882 were associated with poor response to antidepressant treatment or non-remission. Further insights into the role FKBP5 plays in development and antidepressant treatment response may be aided by future studies focused on individuals who previously experienced childhood trauma and later developed MDD.
Keywords: Pharmacogenetics; Depressive Disorder; Major; Adult Survivors of Child Adverse Events (Source: MeSH-NLM).
In 2018, approximately 17.7 million adults in the United States had suffered at least one major depressive episode,1 and a survey conducted by the Centers for Disease Control and Prevention (CDC) estimated 12.7 percent of Americans aged 12 and over took antidepressant medication within the past month.2 Antidepressants used to treat Major Depressive Disorder (MDD) have historically varied in efficacy for different individuals. Prescription of these medications is often on a trial-and-error basis, potentially delaying effective treatment for patients.
In an effort to improve the selection of an effective treatment, antidepressant pharmacotherapy has become one of the first ventures into pharmacogenetics, a field that aims to identify ideal therapies based on the patient's genetic data. Companies including Myriad Genetics have developed genetic testing (GeneSight) to identify genetic polymorphisms that can be used to clinically predict antidepressant efficacy, and are currently being used in practice today. However, the research linking genetic polymorphisms to MDD and antidepressant efficacy have not yielded consistent results.3-5 Support from professional organizations including the American Psychiatric Association is lacking,6 suggesting further study in defined subpopulations may be necessary. Traditionally, studies have used race and ethnicity. Recent studies indicate that there may be several origins and subtypes of MDD, suggesting that a more appropriate subpopulation for genetic studies may be identified by a shared experience or other factors contributing to MDD development.7,8
Increased hypothalamic-pituitary-adrenal (HPA) axis activity has been linked to depression in several studies.9,10 The glucocorticoid receptor plays a critical role in preventing a continued stress response mediated by the HPA axis after a threat has ended.11 FK506 binding protein 5 (FKBP5) is a gene for a glucocorticoid receptor binding protein that regulates glucocorticoid receptor sensitivity within the HPA axis. It has been identified as a probable predictor of antidepressant response and has a potential defined subpopulation for study, individuals who have experienced childhood trauma. FKBP5 variants located in regions of transcriptional regulation including rs1360780 undergo epigenetic alterations (demethylation) as a result of childhood trauma, and have been associated with MDD.11-14 In the proposed mechanism, decreased methylation of FKBP5 at transcriptional regions leads to upregulation of FKBP5, which decreases glucocorticoid receptor sensitivity by binding to its complex and preventing its translocation to the nucleus. This molecular interaction results in increased resistance and ultimately dysregulation of the HPA axis, predisposing individuals to developing MDD.11,15-20 Additionally, early studies in mice show that loss of FKBP5 reduces expression of excitatory glutamate receptors and increases the expression of GABA in the hippocampus, ultimately affecting long term potentiation and neuroplasticity.21 FKBP5 rs1360780 polymorphisms in individuals with MDD who experienced childhood trauma have also been associated with structural and functional differences within the brain, including reduced activity in the insula following emotional stimuli, and lower FKBP5 methylation levels have been linked with reduced gray matter concentration in the inferior frontal orbital gyrus bilaterally, an area associated with depressive symptoms.22-24 Other studied variants include the rs9296158, rs3800373, and rs9470080 alleles.3,11,12, 25-28
This review will examine the epigenetically-modified FKBP5 variants associated with previously experienced childhood trauma and their potential to predict antidepressant response. Searching the literature for genetic variants associated with a pathophysiologic mechanism may yield insights into future research directions to identify targeted treatments for specific subpopulations.
PubMed and Web of Science were searched for relevant articles. Titles and abstracts were analyzed manually for direct relevance to the hypothesis. Required inclusions were 1) focus on individuals diagnosed with unipolar MDD and 2) associations between FKBP5 variants and response to antidepressant treatment. FKBP5 polymorphisms associated with childhood trauma and development of MDD were of particular interest, but polymorphisms that did not meet these criteria were also included. Other inclusion criteria were human-based studies that specifically researched the FKBP5 gene and its variants and antidepressant treatment response.
Studies that were published prior to 2008, meta-analyses, literature reviews, consensus papers, editorials, and updates were excluded. Additionally, articles that were considered out-of-scope of this review included studies that used non-human models with a focus on neuronal differentiation, studies that focused on the interaction between FKBP5 and other genes, and those that focused on FKBP5 variants and their impact on conditions other than MDD (e.g., anxiety disorders and bipolar disorder, high stress reactivity and stress hormone regulations). Studies that did not differentiate between unipolar and bipolar depression in their patient sample and those that examined FKBP5 and MDD in older adults without a history of childhood maltreatment were also excluded.
An initial search in PubMed for articles published between 2008-2018 with the search string “FKBP5” AND “major depression” yielded 32 results. Of these articles, 31 were excluded: 23 were out of scope as determined by the criteria above, 3 were review articles, 2 were meta-analyses, 2 were updates or reflections, and 1 was an editorial. The search string “FKBP5” AND “depression” AND “pharmacogenetics” yielded 10 results; 7 articles were excluded as 4 were determined to be out of scope, 2 were updates or reflections, and 1 was a meta-analysis. The search string “antidepressant response” AND “FKBP5” yielded 13 results. Nine of these articles were excluded, with 4 out of scope, 2 reviews, 1 meta-analysis, 1 consensus paper, and 1 update. Due to the specificity of the search for the FKBP5 gene and its variants, utilizing MESH terms did not yield results within the inclusion criteria.
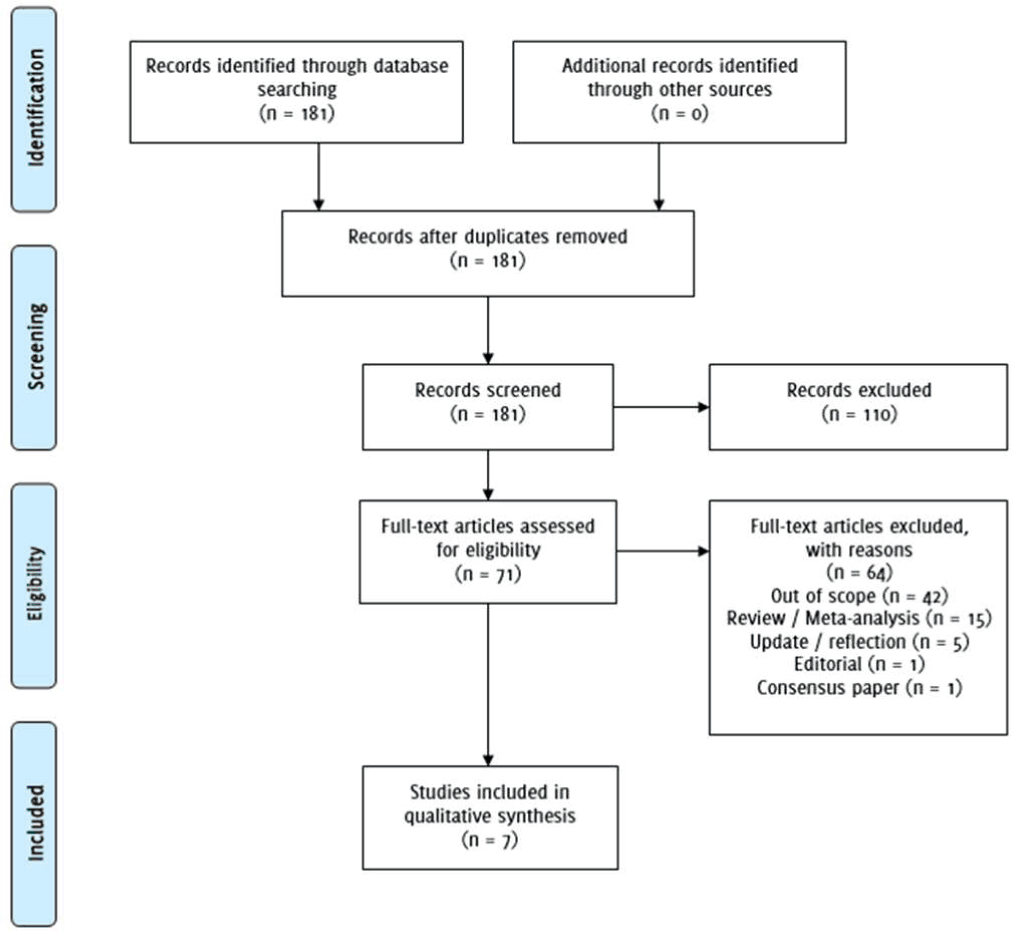
Repeating the searches with Web of Science using “FKBP5” AND “major depression” published between 2008-2018 yielded 112 findings, and one additional article for analysis. Using the search terms “FKBP5” AND “depression” AND “pharmacogenetics” yielded 14 results, and included 3 repeated findings. “Antidepressant response” AND “FKBP5” did not yield additional articles for analysis. In total, 7 articles were selected for inclusion in analysis. Figure 1 describes the PRISMA flow diagram.
Figure 1PRISMA Flow Diagram

For the articles included in the analysis, the study characteristics and methods were extracted. The number of participants, methods used to assess depression symptoms, specific antidepressant treatments or algorithms, and methods used to assess treatment response were noted for each study. Articles were examined for associations between antidepressant response and FKBP5 polymorphisms or mRNA levels. These findings were grouped by FKBP5 expression levels, polymorphisms previously found to be associated with childhood trauma and development of MDD, and polymorphisms that did not have previous evidence of association with childhood trauma and development of MDD.
Cattaneo et al.29 and Banach et al.30 examined FKBP5 expression levels following antidepressant treatment with conflicting results (Table 1). Cattaneo et al.29 selected participants from the GENDEP trial for analysis. Leukocyte mRNA levels of FKBP5 and other candidate genes were measured using quantitative PCR. FKBP5 expression levels were normalized to the mean expression of reference genes including glyceraldehyde 3-phosphate dehydrogenase, beta actin, and beta-2 microglobulin. The Pfaffl method, which accounts for differences in primer efficiencies, was used to compare FKBP5 expression with controls. Additionally, changes in FKBP5 mRNA levels were compared before treatment and after 8 weeks.
Table 1Studies that Examined Antidepressant Response and FKBP5 Gene Expression/Polymorphisms
| Author | Genetic variant or gene expression | Population | MDD criteria | Antidepressant | Assessment of response |
|---|---|---|---|---|---|
| Banach et al. (2017)30 | Leukocyte FKBP5 gene expression | 30 female patients age 18-60 from the Department of Adult Psychiatry at Poznan University of Medical Sciences | DSM-IV and HAM-D Moderate severity |
Participants were drug free at least 6 months prior to entering study Sertraline (50-150 mg) or Venlafaxine (75-225 mg) |
Improvement in depressive symptoms, as determined by >50% reduction in HAM-D Remission determined by HAM-D <8 |
| Cattaneo et al. (2013)29 | Leukocyte FKBP5 mRNA levels | 31 males and 43 females from the GENDEP trial (811 adult outpatients; 296 men and 514 women from 9 psychiatric center in 8 European countries) age 19-72 | DSM-IV Moderate severity |
Selected subset did not take medication for at least 2 weeks prior to entering the
trial Nortriptyline (50-150mg daily) or Escitalopram (10-30 mg daily) for 12 weeks |
Assessed weekly for depression symptom severity and antidepressant response using
MADRS, HAM-D, and BDI Successful response: >50% reduction in MADRS score from baseline through week 12 |
| Lekman et al. (2008)31 | rs4713916 | 1,809 patient samples age 18-75 from STAR*D study that were determined to have MDD Patients were compared to 739 controls that were obtained from the Rutgers Cell Repository and were determined not to have a mental health condition |
DSM-IV | Citalopram as a first-line antidepressant; data up until 14 weeks was used for analysis to account for patients that were resistant and required a second treatment | QIDS-C to measure antidepressant response at each visit and at 14 weeks; patients were determined to have responded to treatment if they had at least a 50% reduction in their QIDS-C score and patients determined to be in remission had a QIDS-C score ≤5 |
| Zobel et al. (2010)32 | rs3800373, rs755658, rs1360780, rs1334894, rs4713916 |
110 German patients age 18-60 with recurrent unipolar depression, as determined by DSM-IV, after accounting for drop-outs and 284 controls | DSM-IV | Tapered off previous antidepressants prior to start of study Citalopram (20-40 mg) with or without Lorazepam |
HAM-D at baseline (Day 8) and Day 36 Antidepressant response assessed using dexamethasone-suppressed corticotropin-releasing hormone test |
| Stamm et al. (2016)33 | rs1360780 | 298 patients age 18-70 with MDD who were recruited from hospitals and were participating in the German Algorithm Project (GAP3) or the Zurich Algorithm Project | DSM-IV and HAM-D score ≥15 | Patients in both studies received a stepwise treatment regimen guided by an algorithm
(n=171) or standard treatment (n=127). Participants first received one of four antidepressant classes (venlafaxine, sertraline, amitriptyline, or reboxetine) and maintained the same dosage for at least 4 weeks. Patients who did not respond (HAM-D score reduction <30%), received lithium, an increased dose of the same medication, or change to a different antidepressant class. Patients who did not respond to the escalated step continued with other options until receiving electroconvulsive therapy. For the standard treatment group, physicians were allowed to choose the appropriate treatment |
Assessed for antidepressant response at the start of the study and every two weeks
throughout the treatment period using HAM-D Patients with a HAM-D score less than 10 were determined to be in remission |
| Fabbri et al. (2018)34 | rs3800373 rs1360780 rs9470080 |
Three original populations:
STAR*D sample was used for replication |
|
First European Sample: Unspecified antidepressants Second European Sample: Venlafaxine then Escitalopram (if no response after 6 weeks) Italian Sample: Unspecified antidepressants |
First European Sample: HAM-D after 4 weeks of treatment Second European Sample: MADRS was used to evaluate each patient's symptoms of depression biweekly from the start of the study through week 12 Italian Sample: HAM-D; evaluated weekly from beginning of the study through week 8 For the three original patient populations, response to antidepressant treatment or remission was evaluated at week 4 or 6; response was determined by a decrease of 50% or greater in the HAM-D or MADRS and remission was a score of less than or equal to 7 on the HAM-D or less than 10 on the MADRS |
| Ellsworth et al. (2013)35 | rs9380514 SNP 35758265 (no rs number available) rs352428 |
512 patients in the Mayo Clinic Pharmacogenomics Research Network-Antidepressant Medication Pharmacogenomic Study (Mayo PGRN-AMPS) and a replication study of 950 patients from the STAR*D study | HAM-D score ≥14 | Citalopram or escitalopram | Treatment response was assessed at 4 and 8 weeks using the QIDS-C, and patients with ≥50% reduction in QIDS-C score were determined to have responded. Patients with a QIDS-C score less than 5 were determined to be in remission |
FKBP5 mRNA levels in depressed patients were found to be higher than in controls (controls (mean±SEM): 0.99±0.02; patients: 1.26±0.02; +27% change, p<0.0001). 51 patients responded to antidepressants, with 26 responding to escitalopram and 25 responding to nortriptyline. 23 patients did not respond to antidepressants, with 12 not responding to escitalopram and 11 not responding to nortriptyline. For all 74 patients, FKBP5 mRNA levels were 1.26±0.02 prior to treatment, and 1.17±0.02 after 8 weeks of treatment. Successful antidepressant treatment, irrespective of drug class, reduced FKBP5 mRNA levels in depressed patients (no errors reported; −11% change, p<0.0001). There was no effect on FKBP5 levels in patients who did not respond to treatment (no errors reported; −2% change, p=0.45; response x time interaction: F=4.4, p=0.04; drug x time interaction: F=0.05, p=0.8).
Banach et al.30 collected peripheral blood for isolation of RNA from leukocytes and a dexamethasone suppression test prior to the start of antidepressant treatment. Eight weeks after receiving treatment, a second blood sample was collected. FKBP5 gene expression was quantified using qPCR with 18S rRNA used as a reference gene. Differences in gene expression before and after treatment were compared using the non-parametric Wilcoxon signed-rank test.
Three patients were observed to have HPA axis dysregulation at the beginning of the study, and two had normal function following 8 weeks of treatment. No statistically significant difference in mean cortisol levels before and after 8 weeks of treatment was found (p=0.944). Following 8 weeks of antidepressant treatment, all participants experienced improvement in depressive symptoms and all but one, were in remission. The mean HAM-D score value was 3.4±3.1 for patients taking sertraline and 5.0±2.4 for patients taking venlafaxine. There was no difference in gene expression of FKBP5 before and after antidepressant treatment (pre-treatment: max=16, upper quartile=15, median=13.2, lower quartile=11.2, min=11; post-treatment: max=16.8, upper quartile=14.4, median=13.1, lower quartile=11.8, min=9.9; p=0.813).
Lekman et al.,31 Zobel et al.,32 Stamm et al.,33 and Fabbri et al.,34 found correlations between rs1360780, rs3800373, rs9470080, and rs4713916 and antidepressant response/non-response and remission.
Lekman et al.31 determined FKBP5 genotype using Illumina Next Generation Sequencing. In the genotype association test to identify associations with antidepressant response, FKBP5 rs4713916 was shown to be significantly associated with remission (AA/GG genotype: OR=1.60, 95% CI: 1.04-2.43; AG/GG genotype: OR=1.47, CI: 1.15-1.88; p=0.049) but not treatment response (p>0.05) when studied in all ethnicities and corrected for multiple testing. Studying linkage disequilibrium patterns reveals that rs4713916 single nucleotide polymorphisms (SNP) may serve as the functional region of FKBP5.
Zobel et al.32 examined five FKBP5 polymorphisms located in the same haplotype block including rs3800373, rs755658, rs1360780, rs1334894, and rs4713916. DNA samples were collected from whole blood and fluorescence-based allelic discrimination techniques were used for analysis. Comparison of FKBP5 genetic variants between the treatment group and controls and mean values of treatment response were compared using t-tests.
FKBP5 variants rs3800373 (AA allele) and rs4713916 (GG allele), also associated with MDD diagnosis, demonstrated a decreased reduction in cortisol secretion after 4 weeks of treatment (rs3800373: pre-treatment mean=2211, errors not given; post-treatment mean=-426, errors not given; p=0.08; rs4713916: pre-treatment mean=1932, errors not given; post-treatment mean=-338, errors not given; p=0.04). These associations were insignificant with multiple testing. No significant changes were found in HAM-D scores.
Stamm et al.33 determined FKBP5 rs1360780 genotypes. Cox regression analysis was used to analyze the interaction between genotype and antidepressant treatment. The FKBP5 rs1360780 C allele carriers were found to benefit from the standardized antidepressant treatment algorithm. Approximately 64.4% of patients undergoing the algorithm treatment reached remission compared to 38.8% of those in the usual treatment group (p<0.001). Patients who were rs1360780 TT allele carriers showed better treatment response with the first treatment step than CT/CC allele carriers (TT: 60.9%, CT/CC: 38.2%, p=0.03), but there was not a significant difference at the end of the treatment sequence (TT: 69.6%, CT/CC: 53.5%, p>0.05). After six weeks of treatment with one antidepressant, rs1360780 TT allele carriers were found to have significant improvement (HR=1.89, p<0.03). Additionally, rs1360780 TT allele carriers were more likely than CC/CT allele carriers to achieve remission (HR=0.52; p=0.01).
Fabbri et al.34 analyzed genetic data from three original patient populations and the patients from the STAR*D sample to study the association of various candidate genes, including FKBP5, with remission, antidepressant response, and treatment resistance. The STAR*D sample, as described previously, was used for replication. Effects of selected SNPs on antidepressant response, remission, and treatment resistance were tested using logistic regression models.
Fabbri et al.34 examined FKBP5 polymorphisms and their association with four phenotypes: response to the treatment provided to the associated population, remission to the provided treatment, lack of response to escitalopram, and lack of remission when taking escitalopram. In the first European sample, FKBP5 rs3800373 CC genotype and C allele were associated with a higher risk of non-response (genotype: p=0.046, OR=0.11, 95% CI: 0.005-0.68). Additionally, the rs3800373 CC genotype corresponded with treatment resistance, including lack of response (p=0.01 OR=5.15, CI: 1.47-18.87) and lack of remission (p=0.02, OR=6.44, CI: 1.51-35.34) when taking escitalopram. The rs3800373 CA genotype was only correlated with lack of remission when taking escitalopram in the second European sample (p=0.04, OR=2.93, CI: 1.07-8.55), and was associated with a higher risk for lack of response (p=0.01 OR=0.28, CI: 0.09-0.73) and remission (p=0.03, OR=0.27, CI: 0.07-0.83) in the ITAS sample. The rs3800373 C allele was linked with higher risk of non-response in the first European sample (p=0.03, OR=0.50, CI: 0.26-0.93) and the second European sample (p=0.02, OR=0.52, CI: 0.29-0.91). The rs3800373 C allele was also associated with lack of response to escitalopram (p=0.005, OR=2.35, CI: 1.29-4.27) and lack of remission when taking escitalopram in the second European sample (p=0.002, OR=3.07, CI: 1.52-6.42).
The rs1360780 TT genotype was associated with a higher risk of non-response to venlafaxine (p=0.02, OR=0.24, CI: 0.065-0.71), lack of response to escitalopram (p=0.01, OR=4.27, CI: 1.40-13.04), and lack of remission when taking escitalopram (p=0.02, OR=4.76, CI: 1.36-19.77) in the second European sample. In the ITAS sample, the rs1360780 CT genotype correlated with a higher risk of non-response to antidepressants (p=0.002, OR=0.22, CI: 0.08-0.55) and non-remission (p=0.003, OR=0.17, (CI: 0.05-0.52). The rs1360780 T allele corresponded with lack of response to venlafaxine in the second European sample (p=0.02, OR=0.60, CI: 0.38-0.93) and treatment in the ITAS sample (p=0.009, OR=0.40, CI: 0.20-0.78). Additionally, in the second European sample, the T allele was associated with lack of response (p=0.01, OR=1.95, CI: 1.16-3.26) and lack of remission (p=0.01, OR=2.05, CI: 1.17-3.60) when taking escitalopram. In the ITAS sample, the T allele was linked with a higher risk of non-remission (p=0.04, OR=0.45, CI: 0.20-0.95).
In the second European sample, the rs9470080 TT genotype was associated with lack of remission when taking escitalopram (p=0.0469, OR=3.96, CI: 1.06-16.65). The rs9368882 TT SNP, found to be in high linkage disequilibrium with rs9470080 (R2=0.67), was correlated with a higher risk of non-remission in patients taking citalopram in the STAR*D replication study (p=0.035, OR=0.82, CI: 0.69-0.98).
Fabbri et al.34 also provided another perspective of their results, as described within this paragraph. All findings showed consistent effects in two or more of the groups that responded to antidepressant treatment, were in remission, or did not respond or achieve remission while taking escitalopram. In the second European sample, rs3800373 AA genotype/A allele showed some evidence of response and remission (no OR or CI reported; p=0.03 for the allelic repeated-time analysis). The rs1360780 CC genotype/C alelle were linked with response to venlafaxine and remission, and confirmed using repeated time analysis (allele: no OR or CI reported, p=0.01 and gen. .otype: no OR or CI reported, p=0.0059). In the ITAS population, the rs1360780 CC genotype/C alelle, rs3800373 AA allele, and rs9470080 CC genotype were associated with antidepressant response and remission (rs1360780 genotype: p=0.014, no OR or CI reported and allele: p=0.001; rs3800373 AA allele: p=0.003, no OR or CI reported; significance for rs9470080 not given).
Ellsworth et al.35 found additional single nucleotide polymorphisms that may play a role in functional regulation of FKBP5. Ellsworth et al.35 utilized Sanger and Illumina Next Generation genetic sequencing to sequence FKBP5 in lymphoblastoid cells. FKBP5 variants were identified using Next Generation sequencing, and 340 SNPs met criteria for analysis including having a minor alley frequency of 1% or higher, passing the Illumina Golden Gate genotyping quality control criteria, and absence of significant deviation from Hardy-Weinberg equilibrium. The effect of different SNPs on response and remission were analyzed using logistic regression models and were adjusted for potential population stratification. Electrophoresis mobility shift and reporter gene assays were performed on nuclear extracts from two glioblastoma cell lines and lymphoblastoid cells.
Association analysis for the Mayo PGRN-AMPS patients found that FKBP5 rs9380514 (A allele) corresponded to poor antidepressant response at the last visit (P=0.0249, OR=0.65 (95% CI: 0.44-0.95)) and after 8 weeks (P=0.0175, OR=0.59 (CI: 0.38-0.91)). SNP 35758265 (no rs number available) corresponded with a percentage change in QIDS-C after the last visit (no mean or SEM provided; Spearman: 0.09, P=0.042). Twenty-two SNPs were affiliated with remission after 8 weeks at the last visit, 15 trans FKBP5 expression quantitative trait loci (eQTL) SNPs corresponded to antidepressant response after 8 weeks or at the last visit, and 6 FKBP5 eQTL SNPs were associated with remission after 8 weeks of treatment or at the last visit; however, none of these associations were found to be significant after correcting for multiple testing. Association analysis replicating 6 SNPs from the STAR*D study found FKBP5 eQTL SNP rs352428 to be associated with poor antidepressant response after 6 to 8 weeks (P=0.05, OR=0.74, CI: 0.54–1.00). There were no associations found between rs1360780, rs3800373, and rs4713916 and antidepressant response (no statistical information provided). Electrophoresis mobility shift and reporter gene assays confirmed that rs352428 (A/G alleles) may play a potential role in regulation of transcription.
This review examined studies that evaluated FKBP5 polymorphisms and expression levels and their association with antidepressant response. Studies that included polymorphisms previously linked to a potential underlying epigenetic mechanism of development of MDD in individuals who experienced childhood trauma were of particular interest. FKBP5 is a promising biomarker due to the multitude of evidence associating a specific environmental factor with increased susceptibility to development of MDD.3,11-15,19,20,25-28
Cattaneo et al.29 and Banach et al.30 examined FKBP5 RNA expression levels following antidepressant treatment. While Cattaneo et al.29 displayed decreased RNA expression following treatment, Banach et al.30 did not observe significant differences. Potential explanations for this disparity, as noted by Banach et al.,30 include the lack of HPA axis dysregulation observed in the studied population. Additionally, Banach et al.30 studied a smaller group of individuals (30 participants) compared to the 74 patients studied by Cattaneo et al.,29 which likely resulted in lower statistical power for the former. Both studies contain relatively small sample sizes, and a larger group of participants may be necessary to observe the true effects of antidepressants on FKBP5 levels. Of note, Banach et al.30 observed a trend of decreased FKBP5 mRNA expression after administration of sertraline and an increase of expression after venlafaxine therapy, although significance was not reached.
Variants of the FKBP5 rs1360780 allele were associated with both successful antidepressant response and remission and non-response. Stamm et al.33 found that the rs1360780 TT allele carriers were more likely to respond to a standardized algorithm antidepressant treatment regimen and experience more remission compared to CT/CC allele carriers. However, a greater percentage of CT/CC allele carriers also achieved remission in the algorithm treatment group compared to treatment as usual. In contrast, Fabbri et al.34 found the TT/CT genotypes to be associated with non-response to antidepressants and lower rates of remission. These findings suggest the mode of treatment is significant, and genotype alone may not account for differences in antidepressant response. Fabbri et al.34 found that the rs1360780 CC genotype/C allele had nominal associations with response to venlafaxine and remission, and decreased risk of treatment resistant depression in a European and ITAS population. The data from these two studies suggest that both the TT allele and CT/CC allele may have a better response to different treatments. The positive association with FKBP5 polymorphisms and response to different treatments strongly supports that it plays some role in treatment response. However, the differences in treatment provided within the two studies makes it difficult to parse out the true impact of genotype on treatment response, and future studies might consider comparing rs1360780 polymorphisms and antidepressant response to a wider range of different treatment options and drug classes, including a placebo, within the same population.
Fabbri et al.34 also found the rs3800373 AA genotype/A allele showed some evidence of response and remission in two populations, and the CC genotype/C allele was associated with non-response and lack of remission. Additionally, the rs9470080 CC allele was associated with antidepressant response and remission, and the TT genotype was correlated with decreased remission in patients receiving escitalopram. Also mentioned by Fabbri et al.34 as an interesting finding, the closely-linked rs9368882 was associated with non-remission in patients who received citalopram. Although some of these findings were positive, Fabbri et al.34 noted the associations were nominal. Previous studies have similarly linked these polymorphisms to increased susceptibility of MDD in individuals who experienced childhood trauma, suggesting a basis for the association.3,28 The utilization of three study populations strengthened the likelihood of finding a true association. However, relatively small sample sizes may have prevented strong significant associations from being discovered.
Zobel et al.32 found associations between the FKBP5 rs3800373 AA genotype and rs4713916 and decreased reduction in cortisol secretion after treatment, suggesting a correlation with non-response to antidepressants. However, no significant changes were found in HAMD scores and these associations were insignificant with multiple testing. Interestingly, both the rs3800373 AA genotype and rs4713916 were also associated with MDD diagnosis in the study. Non-response to antidepressant treatment conflicts with the positive rs3800373 AA genotype association found by Fabbri et al. .34 However, the difference in these findings may be due to small effect sizes, as Zobel et al.32 had approximately half of the study population drop out. It should also be noted that those that tended to drop out had a greater average number of depressive episodes and likely were more treatment resistant. This potentially skews their data toward more favorable results, although none were found. Strengths of the study include the use of a dex/CRH test as a biological indicator of antidepressant response. As noted by Zobel et al.32 and Lekman et al.,31 using standardized survey measures to determine response and non-response may be another explanation for disparities in treatment response found in the same polymorphisms.
Lekman et al.31 found rs4713916 was shown to be significantly associated with remission (p=0.049) but not treatment response when studied in all ethnicities and corrected for multiple testing. Zobel et al.32 also found a lack of associated antidepressant response, but the study methods did not account for associations with remission. The study of linkage disequilibrium patterns by Lekman et al.31 showed that the rs4713916 SNP may serve as the functional region of FKBP5, and additional molecular studies may shed light on its involvement in MDD pathogenesis.
Ellsworth et al.35 found two additional SNPs, rs9380514 in the Mayo PGRN-AMPS study and rs352428 in the STAR*D study, that may play a role in functional regulation of FKBP5. Both polymorphisms were found to be associated with poor antidepressant response. However, the study failed to name the number of responders and non-responders, as well as the QIDS-C score that constituted poor response. Although Ellsworth et al.35 found several SNPs that were associated with response and remission, none were found to be significant after multiple testing. The lack of significance may be attributed to limited sample size. To the author's knowledge, rs9380514 and rs352428 have not been studied in individuals with MDD who experienced childhood trauma, but could potentially be examined in future studies. Establishing a molecular mechanism for rs352428 (A/G alleles), a polymorphism associated with transcriptional regulation including transcription factor binding and silencing, may strengthen evidence for its involvement in MDD and treatment response.
A major limitation of many of the studies included in this review were their reports of nominal observed effects, stating that the lack of statistical power was likely due to small sample sizes and lack of heterogeneity. As noted by Zimmerman et al.28 and Hahle, Merz, Meyners, and Hausch,36 existing genome wide association studies have likely been statistically underpowered to identify FKBP5 associations due to many of the associations being linked with specific environmental factors, including childhood trauma. Additionally, Ising et al.37 notes the importance of considering subgroups that may respond to particular treatments due to shared pathology. Existing studies on molecular mechanisms of the pathogenesis of MDD via increased HPA axis activity point to a particular population for further study, individuals who have experienced childhood trauma.11,14,16-19 Data supporting the association between polymorphisms and treatment response might be strengthened by specifically focusing on these individuals, as there have been demonstrated lower methylation levels at many of the same foci involved in antidepressant response. Both the rs1360780 A/T allele and the CC allele have been associated with susceptibility of MDD in individuals who experienced childhood trauma, and further study in this specific subpopulation may yield findings of greater significance.12,13,28
An additional limitation of the studies is the limited clinical relevance of the data. Specific genotypes have not been associated with successful treatment using a specific antidepressant class. Ideally, FKBP5 polymorphisms could be used to predict what treatment would result in the best response for a specific population of patients. The broad associations in these studies fail to inform treatment decisions in medical care. Another criticism of these studies, particularly the STAR*D trial, is their lack of a placebo treatment option, and therefore it is unknown whether remission would have occurred without antidepressants. Additionally, studies took different approaches to offering treatment. Some utilized a standard algorithm while others allowed physicians prescribing choice. While the presence of associations between FKBP5 and different treatments strengthens the evidence for its use as a potential biomarker, a blinded randomized study looking at different treatment responses to multiple classes of antidepressants would be ideal.
As demonstrated by the number of positive associations with FKBP5 polymorphisms and antidepressant response in various study designs, it is likely that with an appropriate study population significant association can be discovered. Prior research linking both morphological changes within the brain and a probable molecular mechanism due to epigenetic changes suggests individuals who have experienced childhood trauma are an ideal population for future study. Focused future study on shared and newly discovered polymorphisms linking FKBP5 with MDD and antidepressant response may yield findings of clinical significance and further personalized psychiatric treatment.
Special thanks to Dr. James P. Bruzik, PhD for his critical review to the manuscript.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: NW, Methodology: NW, Formal Analysis: NW, Investigation: NW, Writing – Original Draft: NW, Writing – Review & Editing: NW & AB, Supervision: AB.
1.Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Rockville, MD: Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data. Last updated: 2019 August 20, Cited: 2020 May 13.
2.Pratt LA, Brody DJ, Gu Q. Antidepressant Use among Persons Aged 12 and Over: United States, 2011-2014. NCHS Data Brief. Number 283. National Center for Health Statistics. 2017.
3.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004 Dec;36 (12):1319.
4.Rao S, Yao Y, Ryan J, Li T, Wang D, Zheng C, Xu Y, Xu Q. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: a comprehensive meta-analysis. Sci Rep. 2016 Sep 7;6:32687.
5.Tsai SJ, Hong CJ, Chen TJ, Yu YW. Lack of supporting evidence for a genetic association of the FKBP5 polymorphism and response to antidepressant treatment. Am J Med Genet B Neuropsychiatr Genet. 2007 Dec 5;144 (8):1097–8.
6.Zeier Z, Carpenter LL, Kalin NH, Rodriguez CI, McDonald WM, Widge AS, Nemeroff CB. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am J Psychiatry. 2018 Sep 1;175 (9):873–86.
7.Ising M, Scheuer S, Belcredi P, Uhr M, Holsboer F. S74. Perspective of Personalized Drug Treatment in Depression. Biol Psychiatry. 2018 May 1;83 (9):S375–6.
8.Tokuda T, Yoshimoto J, Shimizu Y, Okada G, Takamura M, Okamoto Y, Yamawaki S, Doya K. Identification of depression subtypes and relevant brain regions using a data-driven approach. Sci Rep. 2018 Sep 20;8 (1):14082.
9.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000 Nov;23 (5):477.
10.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008 Sep 1;31 (9):464–8.
11.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat Neurosci. 2013 Jan;16 (1):33.
12.Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011 Sep;36 (10):1982.
13.Lavebratt C, Åberg E, Sjöholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord. 2010 Sep 1;125 (1-3):249–55.
14.Tyrka AR, Ridout KK, Parade SH, Paquette A, Marsit CJ, Seifer R. Childhood maltreatment and methylation of FK506 binding protein 5 gene (FKBP5). Dev Psychopathol. 2015 Nov;27(4pt2):1637–45.
15.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009 Dec 1;34:S186–95.
16.Farrell C, Doolin K, O'Leary N, Jairaj C, Roddy D, Tozzi L, Morris D, Harkin A, Frodl T, Nemoda Z, Szyf M. DNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic–pituitary–adrenal axis activity and to early life emotional abuse. Psychiatry Res. 2018 Jul 1;265:341–8.
17.Matosin N, Halldorsdottir T, Binder EB. Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: the FKBP5 model. Biol Psychiatry. 2018 May 15;83 (10):821–30.
18.Menke A, Lehrieder D, Fietz J, Leistner C, Wurst C, Stonawski S, Reitz J, Lechner K, Busch Y, Weber H, Deckert J. Childhood trauma dependent anxious depression sensitizes HPA axis function. Psychoneuroendocrinology. 2018 Dec 1;98:22–9.
19.Vermeer H, Hendriks-Stegeman BI, van der Burg B, van Buul-Offers SC, Jansen M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: a potential marker for glucocorticoid sensitivity, potency, and bioavailability. J Clin Endocrinol Metab. 2003 Jan 1;88 (1):277–84.
20.Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene–stress–epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016 Jan;41 (1):261.
21.Qiu B, Xu Y, Wang J, Liu M, Dou L, Deng R, Wang C, Williams KE, Stewart RB, Xie Z, Ren W. Loss of FKBP5 Affects Neuron Synaptic Plasticity: An Electrophysiology Insight. Neuroscience. 2019 Mar 15;402:23–36.
22.Grabe HJ, Wittfeld K, Van der Auwera S, Janowitz D, Hegenscheid K, Habes M, Homuth G, Barnow S, John U, Nauck M, Völzke H. Effect of the interaction between childhood abuse and rs1360780 of the FKBP5 gene on gray matter volume in a general population sample. Hum Brain Mapp. 2016 Apr;37 (4):1602–13.
23.Tozzi L, Carballedo A, Wetterling F, McCarthy H, O'keane V, Gill M, Morris D, Fahey C, Meaney J, Frodl T. Single-nucleotide polymorphism of the FKBP5 gene and childhood maltreatment as predictors of structural changes in brain areas involved in emotional processing in depression. Neuropsychopharmacology. 2016 Jan;41 (2):487.
24.Tozzi L, Farrell C, Booij L, Doolin K, Nemoda Z, Szyf M, Pomares FB, Chiarella J, O'Keane V, Frodl T. Epigenetic changes of FKBP5 as a link connecting genetic and environmental risk factors with structural and functional brain changes in major depression. Neuropsychopharmacology. 2018 Apr;43 (5):1138.
25.Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. 2005 Dec;10 (12):1058.
26.Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, Mercer KB, Pütz B, Bradley B, Holsboer F, Ressler KJ. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch Gen Psychiatry. 2011 Sep 5;68 (9):901–10.
27.Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010 Jul;35 (8):1674.
28.Zimmermann P, Brückl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, Ising M. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry. 2011 Oct;168 (10):1107–16.
29.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013 Feb;38 (3):377.
30.Banach E, Szczepankiewicz A, Leszczynska-Rodziewicz A, Pawlak J, Dmitrzak-Weglarz M, Zaremba D, Twarowska-Hauser J. Venlafaxine and sertraline does not affect the expression of genes regulating stress response in female MDD patients. Psychiatr. Pol. 2017 Dec 30;51 (6):1029–38.
31.Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, Paddock S. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR* D) Cohort. Biol psychiatry. 2008 Jun 15;63 (12):1103–10.
32.Zobel A, Schuhmacher A, Jessen F, Höfels S, Von Widdern O, Metten M, Pfeiffer U, Hanses C, Becker T, Rietschel M, Scheef L. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int J Neuropsychopharmacol. 2010 Jun 1;13 (5):649–60.
33.Stamm TJ, Rampp C, Wiethoff K, Stingl J, Mössner R, O′ Malley G, Ricken R, Seemüller F, Keck M, Fisher R, Gaebel W. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J Psychopharmacol. 2016 Jan;30 (1):40–7.
34.Fabbri C, Corponi F, Albani D, Raimondi I, Forloni G, Schruers K, Kasper S, Kautzky A, Zohar J, Souery D, Montgomery S. Pleiotropic genes in psychiatry: Calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog Neuropsychopharmacol Biol Psychiatry. 2018 Feb 2;81:203–10.
35.Ellsworth KA, Moon I, Eckloff BW, Fridley BL, Jenkins GD, Batzler A, Biernacka JM, Abo R, Brisbin A, Ji Y, Hebbring S. FKBP5 genetic variation: association with selective serotonin reuptake inhibitor treatment outcomes in major depressive disorder. Pharmacogenet Genomics. 2013 Mar;23 (3):156.
36.Hähle A, Merz S, Meyners C, Hausch F. The many faces of FKBP51. Biomolecules. 2019 Jan;9 (1):35.
37.Ising M, Maccarrone G, Brückl T, Scheuer S, Hennings J, Holsboer F, Turck CW, Uhr M, Lucae S. FKBP5 Gene Expression Predicts Antidepressant Treatment Outcome in Depression. Int J Mol Sci. 2019 Jan;20 (3):485.
Natalie Wietfeldt, 1 BS, Saba University School of Medicine, The Bottom, Caribbean Netherlands
Andrew J. Boileau, 2 PhD, Full Professor, Saba University School of Medicine, The Bottom, Caribbean Netherlands
Mihnea-Alexandru Găman, Editor
Madeleine Jemima Cox, Student Editor
About the Author: Natalie Wietfeldt is currently a medical student of Saba University School of Medicine, Saba, Dutch Caribbean, Class of 2021. She was inducted into the Alpha Omega Phi honor society in September 2018.
Correspondence: Natalie Wietfeldt, Address: The Bottom, Caribbean Netherlands. Email: n.wietfeldt@saba.edu
Cite as: Wietfeldt N, Boileau AJ. FKBP5 Gene Variants as Predictors for Antidepressant Response in Individuals with Major Depressive Disorder Who Have Experienced Childhood Trauma. A Systematic Review. Int J Med Students. 2020;8(2):126-134.
Copyright © 2020 Natalie Wietfeldt, Andrew J. Boileau
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 8, NUMBER 2, August 2020