Review
Next Generation of Advanced Non-Small Cell Lung Cancer Therapy: Targeted and Immuno-Therapies
Sze Wah Samuel Chan1, Elliot Smith1*
doi: http://dx.doi.org/10.5195/ijms.2020.446
Volume 8, Number 1: 26-32
Received 29 11 2019:
Accepted 26 12 2019
ABSTRACT
Lung cancer is one of the deadliest cancers in the world. Current clinical trials
are focused on developing the next generation of therapies that target novel anti-cancer
mechanisms. One approach is to prime the immune system, as the cancer has been known
to suppress immune cells in the tumor microenvironment. Using immunotherapy, the immune
system can be unleashed and suppress the cancer's growth. Another pathway is targeting
known oncogenic genes that are important for the cancer's growth and survival. In
lung cancer, the epidermal growth factor receptor and several other mutated proteins
are targets of small-molecule inhibitors that have been shown to drastically improve
patient survival and quality of life. Discussed in this review are broad highlights
of the different immunotherapies and small molecule targeted therapies that have been
studied in the latest clinical trials for lung cancer.
Keywords:
Lung neoplasms;
Non-Small-Cell Lung Carcinoma;
immunotherapy;
EGFR tyrosine kinase inhibitor;
Molecular targeted therapy (Source: MeSH-NLM).
Introduction
Globally, cancer is increasingly one of the greatest health burdens, with an estimated
20% probability of being diagnosed with cancer before age 75 and a 10% chance of dying
from it.1 According to GLOBOCAN in 2018, lung cancer had the highest incidence, with nearly
2 million cases and the highest mortality at almost 1.7 million deaths. In the United
States, similar trends were observed where 234,030 lung cancer cases were diagnosed,
accounting for 25% of cancer deaths.1
Lung cancer is histologically divided into small cell lung carcinoma (~15% of lung
cancers) and non-small cell lung carcinoma (NSCLC) (85% of lung cancers).2 NSCLC is further subdivided into squamous, adenocarcinoma, and large cell carcinoma.
These histopathological divisions help decide further investigations and management.
Many of the development of the next generation of medical therapies has focused on
stage IV, metastatic lung cancers and will be the focus of this review. Until recently,
the first-line chemotherapy regimen for metastatic NSCLC is chemotherapy with two
drugs, one of which is a platinum agent (known as platinum-doublet chemotherapy);
where appropriate, maintenance chemotherapy with a single agent may take place after
the end of 4-6 rounds of platinum-doublet chemotherapy.3,4 Few effective treatments are available after disease progression and mainly involve
single-agent chemotherapy such as docetaxel.3
For treatment-naïve patients, identification of who benefits from immunotherapy is
contingent on several main factors: lack of a driver mutation (e.g. EGFR or ALK as immunotherapies are likely less effective than targeted therapy in this population)
and biomarker status.5 PD-L1/PD-1 is the most commonly used biomarker status for lung cancer as the approved
therapies all target this immune blockade.6 The PDL1/PD-1 axis is a mechanism by which cancer cells induce T cell anergy and
exhaustion, and avoid anti-tumor immune cells. PD-L1/PD-1 antibodies block this interaction
and allow the immune system to recognize and then suppress the cancer cells.7 The goal of this review is to provide a broad yet comprehensive overview of the different
next-generation cancer therapies in NSCLC and provide a summary of the evidence rationalizing
each therapy. The following section will focus on immune modulators that re-educate
the immune system to attack the cancer cells based on phase III clinical trials data.
The second section will discuss targeted therapies for lung cancers with driver mutations.
Finally, the last section will propose an algorithm for treatment decision making
based on the current evidence.
The adverse events and clinical trials described in this review use the Common Terminology
for Adverse Events (CTCAE) terminology system which aims to unify the nomenclature
in assessing the severity of each adverse event. Grade 1 represents mild adverse events
and intervention is not needed to grade 4 where there are life-threatening consequences
requiring immediate intervention.8,9 Grade 5 is reserved for cases where the adverse event led to death. An example of
a grade 1 event in vomiting means it requires no intervention a grade 2 event is determined
by the need for outpatient IV hydration, a grade 3 event requires hospitalization
or tube-feeding and grade 4 carries life-threatening consequences. Table 1 has examples of different common adverse events reported in oncology clinical trials
and their grading definitions.
Table 1.
Common Terminology for Adverse Events (CTCAE) v5.0 examples.
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
| Rash – Maculo-papular |
| <10% Body surface area (BSA) |
10-30% BSA, limiting activities of daily living, or >30% BSA but no or mild symptoms
of burning, pruritis, or tightness
|
>30% BSA with moderate to severe symptoms; limiting self-care ADLs |
Grade not available |
| Diarrhea |
| <4 stools per day over baseline, mile increase in ostomy output |
Increase of 4-6 stools per day over baseline, a moderate increase in ostomy output,
limits instrumental ADL
|
Increase of >7 stools per day over baseline, hospitalization is indicated, severe
increase in ostomy output, limiting self-care ADLs
|
Life-threatening, urgent intervention required |
| Neutropenia |
| <LLN including 1500/m3, 1.5 × 109 / L
|
<1500 − 1000/mm3, <1.5 − 1.0 × 109 / L
|
<1000 − 500/mm3, <1.0 − 0.5 × 109 / L
|
<500/mm3, <0.5 × 109 / L
|
| Anemia |
| Hemoglobin < LLN, including <10.0 g/dL, <6.2 mmol/L, <100 g/L |
Hemoglobin <10.0-8.0 g/dL, <6.2-4.9 mmol/L, <100-80 g/L |
Hemoglobin <8.0 g/dL, <4.9 mmol/L, <80 g/L; transfusion is indicated |
Life-threatening, urgent intervention required |
Immunotherapy with Monotherapy
Nivolumab as second-line monotherapy for metastatic NSCLC
Nivolumab is a human IgG4 anti-PD-1 monoclonal antibody and has been approved for
second-line therapy regardless of PD-L1 status. In the CheckMate 017 and CheckMate
057 trials, nivolumab was compared to docetaxel for patients with disease progression
after first-line therapy for both squamous and non-squamous histology. Nivolumab lowered
the risk of death compared to docetaxel in squamous (hazard ratio (HR) 0.59, 95% confidence
interval (CI) 0.44 to 0.79, P <0.001, minimum follow-up was 11 months) and non-squamous
(HR 0.73, 95% CI 0.59 to 0.89, P = 0.002, minimum follow-up was 13.2 months) cell
types.10,11 CheckMate 026 evaluated nivolumab monotherapy in the first line, which showed non-significant
differences in overall and progression-free survival.12 However, the nivolumab group reported 18% grade 3-4 adverse events as opposed to
51% in the standard chemotherapy group, mainly with more fatigue and hematological
adverse events such as neutropenia, thrombocytopenia, and anemia in the chemotherapy
group.12
Atezolizumab monotherapy as second-line therapy for metastatic NSCLC
Atezolizumab is a humanized monoclonal IgG1 PD-L1 antagonist. The OAK trial demonstrated
in second-line therapy that atezolizumab improved overall survival (OS) (HR 0.73,
95% CI 0.62 to 0.87, P = 0.0003) compared to docetaxel with a median follow-up of
21 months.13 Similar to nivolumab, the difference was significant regardless of PD-L1 tumor expression.
Fewer grade 3-4 adverse events were observed with immunotherapy (15% vs. 43%), where
chemotherapy had more hematological adverse events such as anemia, neutropenia, and
febrile neutropenia.13
All definitions were extracted directly from the Common Terminology for Adverse Events
(CTCAE) v5.0, developed by the National Cancer Institute (NCI) Cancer Therapy Evaluation
Program (CTEP) published in November 2017, https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm). Each adverse event is graded according to severity, where grade 1 is the least
severe with no intervention to grade 4, which requires urgent intervention and grade
5 is reserved for deaths related to the adverse event. Self-care activities of daily
living (ADL), according to CTCAE, refer to bathing, dressing, undressing, feeding
self, using the toilet, taking medication and not bedridden. Instrumental ADLs refer
to preparing meals, shopping for groceries or clothing, using the telephone or managing
money.
Pembrolizumab as monotherapy in first- or second-line metastatic NSCLC
Pembrolizumab, a humanized monoclonal IgG4 PD-1 receptor antagonist, was first tested
in 2016 where the Keynote 10 trial compared pembrolizumab vs. docetaxel in the second-line,
which showed superior overall survival (HR 0.61, 95% CI 0.49 to 0.75, P <0·0001, median
follow-up was 13.1 months) driven mainly by patients with PD-L1 immunohistochemical
staining of at least 50% (OS HR 0.50, 95% CI 0.36-0.70,P <0.0001) for which it was
mainly powered to detect.14 In the same year, Keynote 24, with a median follow-up of 11.2 months, confirmed the
benefit in the · 50% PD-L1 setting now in the first-line setting. Pembrolizumab treatment
induced longer overall survival (HR 0.60, 95% CI 0.41 to 0.89, P = 0.005) compared
to platinum-based doublet therapy.15 Furthermore, grade 3-5 adverse events were less in the immunotherapy group (26.6%
vs. 53.3%), which were similar to nivolumab and atezolizumab, where the chemotherapy
group had more hematological suppression. One key point from the trial was that immunotherapies
demonstrated more immune-related adverse events such as more severe skin reactions,
pneumonitis and colitis.15 Keynote 42 was a follow-up trial that indicated that tumors with . PD-L1 1% treated
with pembrolizumab showed superior overall survival (HR 0.81, 95% CI 0·71 to 0·93,
P=0·0018) at a median follow-up of 12.8 months with a similar side effect profile
demonstrated in the previous trials.16
Sequential Durvalumab Monotherapy after Chemoradiation in Unresectable Stage III NSCLC
Durvalumab is an IgG1 kappa monoclonal antibody against PD-L1 that was investigated
in the PACIFIC trial in stage III surgically unresectable NSCLC without disease progression
after . 2 cycles of platinum-based chemoradiotherapy compared to placebo. Durvalumab-treated
patients demonstrated superior overall survival (HR 0.68, 99.73% CI 0.47 to 0.997,
P = 0.0025, median follow-up 25.2 months). For grade 3 or 4 adverse events due to
any cause, 30.5% of the durvalumab treatment group reported these events compared
to 26.1% in the placebo group. Most of the grade 3-4 event differences were due to
increased pneumonia and radiation pneumonitis in the immunotherapy group.17,18 Durvalumab is unique as the only approved consolidation immunotherapy single agent
for NSCLC.
Immunotherapy Combination Therapies
Immunotherapy with Chemotherapy for first-line metastatic NSCLC
The latest clinical trials have expanded beyond the monotherapy realm to investigate
more effective front-line therapies. One strategy is to combine chemotherapy and immunotherapy,
and there is evidence to suggest they can work synergistically in melanoma trials.19,20 In the first-line setting, Keynote 189 compared standard platinum-based chemotherapy
± pembrolizumab in non-squamous NSCLC. The immunotherapy with chemotherapy group demonstrated
better overall survival (HR 0.49, 95% CI 0.38 to 0.64, P <0.001) and the trend was
preserved regardless of PD-L1 expression, where median follow-up was 10.5 months.21 Adverse events of grade 3 or higher were found in 67.2% for the combination group
compared to 65.8% for the chemotherapy group. The main difference in grade 3 or higher
events in the combination group was more febrile neutropenia.21 Keynote 407 (median follow-up of 7.8 months), which studied carboplatin + paclitaxel
± pembrolizumab in squamous NSCLC, showed similar improvements for the combination
group (HR 0.56, 95% CI 0.45 to 0.70, P <0.001), with similar number of grade 3 or
higher adverse events (69.8% vs. 68.2%) with more pneumonitis and autoimmune hepatitis
in the combination group.22
One of the CheckMate 227 secondary goals compared first-line platinum-doublet chemotherapy
± nivolumab in the <1% PD-L1 population, which showed improved OS compared to chemotherapy
alone (HR 0.78, 95% CI 0.60 to 1.02, P = 0.035, minimum follow-up was 29.3 months).23 IMpower 131 was a trial comparing first-line carboplatin and nab-paclitaxel ± atezolizumab
in squamous NSCLC, where there was improved progression-free survival (HR 0.715, 95%
CI, 0.603 to 0.848; P = 0.0001) but no differences in overall survival (P = 0.16).24,25 The IMpower130 was a similar trial for non-squamous histological subtypes and reported
at the European Society of Medical Oncology (ESMO) 2018 that atezolizumab + carboplatin
and nab-paclitaxel had superior OS (HR 0.79, 95% CI 0.64 to 0.98, P = 0.033) and PFS
(HR 0.64, 95% CI 0.54 to 0.77; P <0.0001) compared to carboplatin and nab-paclitaxel
alone.26
Dual Immunotherapy
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is another target of immunotherapy
blockade, which combined with PD-L1 blockade, has demonstrated improvement in metastatic
melanoma.27 The rationale is to target different T-cell activation pathways to induce a more
robust tumor response. In CheckMate 227, another secondary goal was to compare nivolumab
and ipilimumab treatment with nivolumab monotherapy in PD-L1 .1%. This comparison
showed trends towards benefit for the PD-L1 .1% population (HR 0.90, 95% CI 0.76-1.07)
and PDL1 .50% (HR 0.87, 95% CI 0.68-1.12), but there were more grade 3-4 events in
the dual immunotherapy group compared to the monotherapy group (35.5% vs. 19.4%).
More specifically it included more rash, pruritis, diarrhea and adverse events with
suspected immune etiology affecting the pulmonary, gastrointestinal, skin and endocrine
systems.23
The MYSTIC trial investigated patients with PD-L1 . 25% with a durvalumab and tremelimumab
(anti-CTLA-4) combination in the first-line setting. Unfortunately, the immunotherapy
combo was unsuccessful in showing PFS or OS improvements over chemotherapy but had
less grade 3-4 treatment adverse event profiles (22.1% in combination vs. 33.8% in
chemotherapy).28 The NEPTUNE trial (durvalumab + tremelimumab vs. chemotherapy) includes ALK and EGFR
patients, and is set to reveal results soon.29
Immune-Related Adverse Events in Immunotherapies
Immunotherapies are not harmless despite a safer side effect profile compared to chemotherapy.
Physicians caring for patients undergoing immunotherapies must be keenly aware of
the unique and dangerous side effects compared to chemotherapy patients (Table 2). The unique danger in immune-oncology is an overstimulation of the immune system,
which can induce an autoimmune condition. The most common immune-related adverse event
(irAE) is dermatological rash/pruritis, with an estimated prevalence of 30% in nivolumab
or pembrolizumab patients.30 Grade 1 toxicities do not necessarily necessitate stopping treatment and can be treated
with topical steroids or symptomatic systemic treatment. Serious irAEs that require
clinicians to be vigilant about are colitis, pneumonitis, endocrinopathies, neurological
syndromes, and cardiovascular compromise (Table 2). For all toxicities, grade 1 events should be monitored for progression while grade
2 and above may necessitate withholding treatment or permanently discontinuing treatment
and immunosuppressive treatment depending on the specific irAE.31 Specific irAE risk stratification and treatment strategies are summarized in the
American Society of Clinical Oncology Clinical Practice Guidelines and the Society
for Immunotherapy of Cancer (SITC) Toxicity Management Working Group.30,31 Due to the potential for high morbidity and mortality associated with pneumonitis,
colitis, neurological syndromes and cardiovascular toxicity, these warrant consideration
of immediate discontinuation and referral to additional specialists. The mainstay
of systemic treatment for grade 2 and above adverse events is corticosteroids, such
as prednisone at 0.5-2 mg/kg/day depending on the grade of the toxicity, along with
appropriate prophylaxis therapies.31 If refractory, immunomodulatory therapies could serve as the next step; for instance,
infliximab has been reported in the use of refractory colitis.31–33
Table 2.
Potential Adverse Events for Immunotherapies for Oncology Treatment.
| Common Adverse Events |
Uncommon Adverse Events |
Indications to Stop Treatment |
| Maculopapular rash |
Renal involvement |
Pneumonitis |
| Pruritis syndromes |
Neurological |
Colitis |
| Fatigue |
Exocrine pancreas involvement |
Neurological syndromes |
| Infusion-related reactions |
Cardiovascular toxicity |
Potentially for cardiovascular toxicity |
| Diarrhea |
Hematological related |
Grade 2-4, reassessment for restarting treatment after symptom resolution |
| Hepatitis |
Ophthalmological inflammation |
|
| Hypothyroidism |
Myositis |
|
| Hypophysitis |
Pneumonitis |
|
| Arthralgia |
Colitis |
|
Legend: The table was adapted from the Society for Immunotherapy of Cancer (SITC) Toxicity
Management Working Group, reference 31: Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities
associated with immune checkpoint inhibitors: Consensus recommendations from the Society
for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother
Cancer. 2017;5(1):1–28.
Targeted Agents for NSCLC Patients Carrying Actionable Mutations
Epidermal Growth Factor Receptor
Epidermal Growth Factor Receptor (EGFR), a receptor tyrosine kinase responsible for
cell proliferation and survival, is one of the most commonly identified driver mutations
in NSCLC. The prevalence of EGFR aberrations in NSCLC varies drastically based on
geographical distribution, varying from 10% in North American and Western European
cases to 30% in East Asian cases.34,35 Studies have reported a higher prevalence in females and non-smokers.34,36 From a histologic perspective, it is most frequently seen in adenocarcinomas compared
to any other NSCLC histologic subtype, with a rate of 30% compared to 2% of another
diagnosis.35,36 The most common of these intracellular TK mutations include the L858R mutation in
exon 21, or short in-frame deletions in exon 19, which both contribute 90% of the
aberrations in this domain.
Gefitinib
Gefitinib is an EGFR inhibitor that competitively inhibits ATP. It is approved for
first-line therapy of EGFR+ metastatic NSCLC. The IRESSA Pan-Asian Study (IPASS) was
a seminal phase III trial demonstrating remarkable efficacy. In the EGFR+ subgroup,
gefitinib was superior to carboplatin-paclitaxel, with an improvement in PFS (HR 0.48,
95% CI 0.36-0.64, P<0.001) and overall survival (HR 0.78, 95% CI, 0.50-1.20).37 Median follow-up in this study was 527 days. In the EGFR-subset, patients treated
with the gefitinib showed a worse PFS (HR 2.85, 95% CI 2.05-3.98, P<0.001).37 From a toxicity perspective, in IPASS, gefitinib was associated with fewer grade
3-4 adverse events (28.7% vs 61.0%).37 The most common toxicities for gefitinib included rash, diarrhea and mucositis, while
neurotoxicities and hematologic toxicities were significantly lower compared to chemotherapy.37
Erlotinib
Erlotinib, like gefitinib, is an EGFR inhibitor and has a similar mechanism to gefitinib.
It is approved for locally advanced or metastatic EGFR+ NSCLC. In the EURTAC trial,
erlotinib was compared to cisplatin-docetaxel or gemcitabine in a first-line setting
and found to have a significantly improved PFS (HR 0.16, 95% CI 0.10-0.26, P<0.0001).38 The median follow-up in this study was 18.9 months in the erlotinib group. Other
phase III trials using erlotinib in the first-line setting compared against platinum-based
combination chemotherapy regimens, such as OPTIMAL and ENSURE, have shown similar
results with erlotinib achieving a significant improvement in PFS.39,40 Erlotinib has also been studied as a combination therapy through the FASTACT trial
where erlotinib intercalated with gemcitabine and platinum therapy versus chemotherapy
with placebo was compared in patients with NSCLC. The erlotinib combination, in EGFR+
mutated NSCLC, showed a significant increase in PFS (HR 0.25, 95% CI 0.16-0.39, P<0.0001).41 Erlotinib intercalated chemotherapy also showed significant improvements in overall
survival (HR 0·48, 95% CI 0.27–0.84, P = 0·0092).41 Median follow-up in the erlotinib plus chemotherapy was 28.2 months. In the EURTAC
trial, erlotinib had fewer grade 3-4 adverse events than chemotherapy (45% vs 65%).
Rash and elevated aminotransferase concentrations were the most frequent adverse events
in the erlotinib group compared to neutropenia and anemia being the most frequent
adverse events in the chemotherapy group.38
Afatinib
Afatinib is a second-generation EGFR tyrosine kinase inhibitor (TKI) and exhibits
its effect by irreversibly binding to the kinase domain in all erbB family tyrosine
kinases. LUX-LUNG-1 was a phase IIB/III trial that studied afatinib versus placebo
in NSCLC patients who have previously received chemotherapy and have failed on a previous
EGFR TKI. While there was no significant difference in OS, PFS was improved (HR 0.38,
95% CI 0.31–0.48, P<0·0001) with afatinib, and these patients were followed for an
estimated two years.42 In addition, in patients who previously developed resistance, PFS was significantly
improved by afatinib treatment, which supports its role as a treatment-following resistance
development in first-line EGFR TKIs. Adverse events were similar between afatinib
and chemotherapy, where the most frequently encountered events include rash, diarrhea
and mucositis. In LUX-LUNG-8, afatinib showed improved PFS and OS compared to erlotinib.43
Osimertinib
Osimertinib is a third-generation EGFR TKI and, similar to afatinib, acts by binding
irreversibly to the EGFR receptor. Intriguingly, this drug shows a pharmacological
effect in patients with sensitizing mutations such as exon 19 deletion or exon 21
L858R as well as patients with the exon 21 T790M mutation, typically conferring resistance
to EGFR TKIs. In the AURA3 trial, osimertinib was compared to platinum-pemetrexed
in patients who had previously failed on first-line EGFR TKI. Osimertinib showed superior
progression-free survival (HR 0.30, 95% CI 0.23-0.41, P<0.001) and fewer grade 3-4
adverse events than chemotherapy (23% vs 47%). Median follow-up for all patients in
this study was 8.3 months. The most common side effects within the osimertinib group
were paronychia, rash and diarrhea compared to anemia and GI-related adverse events,
which were the most common toxicities seen in the chemotherapy group.44 The FLAURA trial showed osimertinib provided a significant improvement in progression-free
survival versus gefitinib or erlotinib in the first-line setting (HR 0.46, 95% CI,
0.37-0.57, P<0.001). Median follow-up for the osimertinib group was 15 months and
9.7 months in the standard EGFR-TKI group.45 The drug has recently been approved for first-line treatment of metastatic EGFR+
NSCLC.
NSCLC patients carrying Anaplastic Lymphoma Kinase translocations
Anaplastic Lymphoma Kinase (ALK) is a receptor tyrosine kinase normally found on chromosome
2. Under normal circumstances, it is expressed at low levels in the small intestines,
neural tissue, male testes and has a role in neural development.46 It is found mutated and fuses with other partner genes in approximately 1-5% of NSCLC
cases and, similarly to EGFR, is found in predominantly non-smokers and adenocarcinoma
histology.47
Crizotinib
Crizotinib is a TKI that inhibits ALK. PROFILE-1007 was a phase III study comparing
crizotinib to chemotherapy in ALK-positive NSCLC following failure on the first-line,
platinum-based doublet chemotherapy. Crizotinib showed a significant PFS improvement
to chemotherapy (HR 0.49, 95% CI 0.37-0.64, P<0.001).48 The median follow-up in the crizotinib group was 12.2 months. Crizotinib also showed
a more tolerable toxicity profile compared to chemotherapy, where the most common
adverse events were visual disorder, GI-related symptoms, and elevated aminotransferase
levels. PROFILE 1014 studied crizotinib in chemotherapy-naïve ALK-positive NSCLC and
found PFS to be significantly improved in the crizotinib group compared to chemotherapy
(HR 0.45; 95% CI 0.35-0.60, P<0.001, median follow-up of 17.4 months).49
Ceritinib
Ceritinib is a next-generation TKI approved for ALK mutated NSCLC. As a more potent
inhibitor, ceritinib has displayed activity in certain cases that have developed resistance
and progressive disease on crizotinib. The ASCEND-1 trial studied ceritinib in patients
who were previously treated and progressed on crizotinib, and found that 56% responded
(95% CI, 49-65%), and the median PFS was 6.9 months (95% CI, 5.6-8.7 months). Median
follow-up was 11.1 months. The most common adverse events included GI-related symptoms,
primarily grade 1 or 2 in severity, including elevated aminotransferase concentrations.50
Alectinib
Alectinib is another next-generation ALK-targeted TKI that has shown activity against
crizotinib-resistant NSCLC. Of particular interest, this drug is not a P-Glycoprotein
substrate and therefore, can achieve significantly improved intracranial concentrations
for targeting CNS metastases. Alectinib was then compared to crizotinib in the first-line
setting in two clinical trials, J-ALEX and ALEX, with improved PFS for alectinib and
12-month event-free survival rate, which was 68.4% vs. 48.7% (95% CI, 61-76% vs. 40-57%).51 Furthermore, CNS progression occurred in 12% of the alectinib group compared to 45%
of the crizotinib group. Median follow-up in this study was 17.6 months in the crizotinib
group and 18.6 months in the alectinib group. Overall, alectinib had more adverse
events than crizotinib; however, when comparing based on severity, alectinib had marginally
fewer grade 3-5 adverse events (41% vs 50%). The most common adverse events in the
alectinib group include GI-related symptoms, elevated aminotransferase concentrations,
peripheral edema and myalgia. Most impressively, the ALEX trial found a PFS of over
34 months compared to 12 months in patients treated with crizotinib, setting the stage
for a change in first-line recommendations to alectinib soon.51
Brigatinib
Brigatinib is an additional next-generation ALK TKI that has shown activity in this
NSCLC subtype. In the ALTA-1L phase III trial, brigatinib was compared to crizotinib
in a front-line setting. Median follow-up in this trial was 11 months in the brigatinib
group and 9.3 months in the crizotinib group. Brigatinib was found to be more efficacious
for PFS (HR 0.49, 95% CI 0.33-0.74, P<0.001).52 In addition, brigatinib was found to show increased activity against CNS lesions
with an observed response rate of 78% (95% CI, 52-94%) compared to 29% (95% CI, 11-52%)
in the crizotinib group.52 The brigatinib group had a higher frequency of grade 3-5 adverse events, occurring
in 61% of patients compared to 55% of the crizotinib group, the most common of which
were elevated creatine kinase, elevated lipase levels and hypertension.52
Treatment Algorithm for Advanced NSCLC
The treatment of patients with metastatic or unresectable NSCLC is rapidly evolving
and increasingly complex based on the most available data. The immunotherapy summary
is adapted from the expert opinion provided by the Society for Immunotherapy of Cancer
2018 NSCLC consensus statement as well as the compilation of the targeted therapy
trials highlighted above (Figure 1).53
Figure 1.
Treatment Algorithm for Advanced NSCLC.
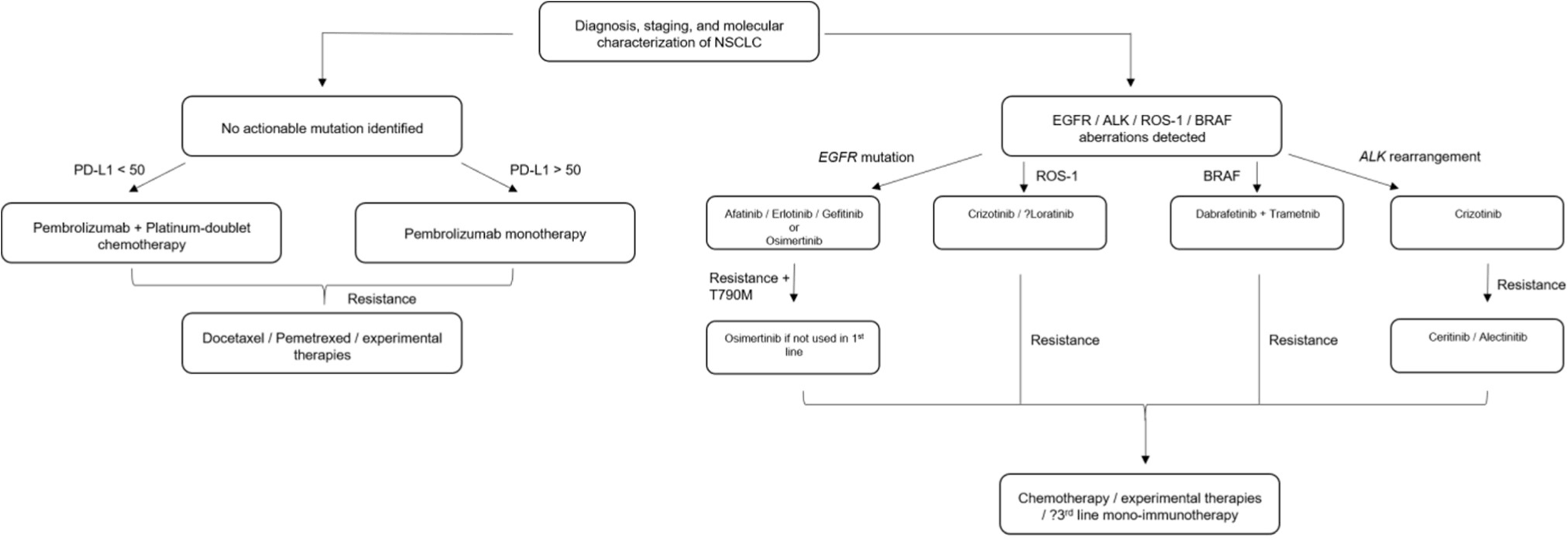
Legend: The treatment of patients with advanced NSCLC is detailed here in the first-and second-line
setting. Patients with no identifiable driver mutations follow along the treatment
course on the left, while patients with EGFR/ALK/BRAF/ROS1 molecular alterations follow
the right side. Patients with NSCLCs that carry BRAF and NTRK molecular alterations
were excluded from this algorithm as there is not enough evidence to suggest a straightforward
treatment path and will be up to the discretion of the treating physician based on
evidence discussed above. All treatment decisions should be tailored to a patient's
specific performance status, biomarkers, prior treatments and the physician's clinical
judgement. Parts of the figures were adapted from the Society of Immunotherapy, see
reference 53, Herbst RS, Davies MJ, Neal JW, Sagorsky S, Gandhi L, Antonia SJ, et al. and The
Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment
of non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6(1):1–15), while
the targeted therapy part was adapted from the studies in this paper.
With the diagnosis of advanced NSCLC, standard staging should first be conducted if
not already completed. Following that, driver mutation analysis, histological subtype
and PD-L1 expression are some of the most basic molecular profiling to decide on initial
treatment decisions.
For patients with non-squamous histology and an actionable driver mutation, TKI therapy
would be initiated first in almost all cases. For EGFR positive patients, afatinib,
erlotinib, gefitinib and osimertinib may be used in first-line with osimertinib used
in treatment-resistant cases. For ALK-fusion tumors, crizotinib is considered first-line
with ceritinib and alectinib following progression; however, this is likely to change
soon given the superiority of alectinib in both efficacy and toxicity profiles in
the first-line setting because of the ALEX trial results. Also, upcoming trial data
of lorlatinib, a fifth ALK inhibitor, has some preliminary evidence of efficacy in
patients who have failed multiple other ALK inhibitors; however, phase III trial data
is not yet available.54
For tumors carrying ROS1 fusions, there is evidence that crizotinib and lorlatinib
are effective; entrectinib is another drug that is showing promise.55,56 For BRAF-mutated tumors, dabrafenib and trametinib can be considered.
For patients who have failed targeted therapy, platinum doublet therapy should be
considered as second-line therapy due to previous poor efficacy of immunotherapy in
this subpopulation.53 Third-line would include immunotherapy monotherapy, including atezolizumab, nivolumab
or pembrolizumab. Patients with no identifiable, actionable driver mutation have the
choice for pembrolizumab/immunotherapy alone or a combination for first-line therapy.
While Checkmate 227 showed some initial promising data for dual-immunotherapy vs monotherapy,
it was not powered enough to detect a meaningful difference, and there were some initial
concerns of immune-related adverse events. For patients with PD-L1 < 50%, there is
evidence for pembrolizumab with chemotherapy for both squamous (carboplatin + nab-paclitaxel
or paclitaxel) and non-squamous cell histology (platinum + pemetrexed). For PD-L1
. 50% non-squamous cell, there is benefit from either pembrolizumab monotherapy or
pembrolizumab + chemotherapy that is dependent on symptomology and how fast the cancer
is progressing. For PD-L1 . 50% in squamous cells, there are similar choices but less
strong evidence for combination therapy. Pending the peer-reviewed published results
of IMpower 130, atezolizumab + carboplatin and nab-paclitaxel may be an alternative
option for squamous cell carcinomas.
For patients who progress on immunotherapy, the second-line would include either pemetrexed
(non-squamous) or docetaxel (squamous) based on standard second-line chemotherapy
regiments. Furthermore, with patients with isolated sites of progression or “oligoprogression,”
there is a consideration for local therapy, but the management is not within the scope
of this review.
Conclusion
In summary, immunotherapies have transformed metastatic lung cancer care by providing
more durable responses and more effective responses compared to traditional chemotherapy.
There is no doubt about the clinical benefit of immunotherapies; however, the current
question is identifying the patients who would benefit. Only 40-50% of PD-L1 positive
patients clinically benefit from immunotherapy, while 15% of PD-L1 negative patients
also benefit.57 This highlights the complexity of the mechanism of these immunomodulatory drugs that
are not explained fully by PD-L1 expression. Tumor mutational burden, cytotoxic T
cell infiltration, immune gene signatures and immune compositive biomarkers are emerging
biomarkers.58 For targeted therapies, the race between drug targets and resistance will continue,
and novel strategies for identifying resistance and combination therapy will be the
key to future success in controlling these oncogene-addicted cancers.
Given the substantial survival benefit with minimal side effect profile of targeted
therapies, there should be a greater awareness in the medical education community
to understand that even poor functional status patients with actionable mutations
can benefit from these small molecule inhibitors. Furthermore, with the paradigm shift
of immunotherapies in oncology where the goal is stimulation of the immune system,
rather than immunosuppression as more classically taught with immune modulators, there
should be greater education in learning about these mechanisms and immune-related
adverse events.
One additional consideration for this new generation of therapies is the cost to the
patient and the healthcare system. There are some studies that suggest that first-line
pembrolizumab and consolidation durvalumab are cost-effective with a similar conclusion
for EGFR+ and ALK translocation directed therapies in NSCLC.59–65 Future studies will help identify patients who can most benefit from these next-generation
therapies and help minimize toxicities and undue financial burden.
Acknowledgments
The authors would like to acknowledge Dr. Geoffrey Liu for his mentorship, critical
review of the manuscript for accuracy and expert guidance.
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions
SWSC and ES were involved in the conceptualization, investigation of data and writing
of the manuscript.
References
1.Ferlay J. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. 2018;1–13.
2.Inamura K. Lung Cancer : Understanding its Molecular Pathology and the 2015 WHO Classification. 2017;7(August):1–7.
3.Brule S. Second Line and Maintenance Therapy for Advanced Non-Small Cell Lung Cancer without
Driver Mutation: An Evolving Paradigm. Int J Cancer Clin Res. 2016 Jun 30;3(3):055.
4.Zarogoulidis K, Zarogoulidis P, Darwiche K, Boutsikou E, Machairiotis N, Tsakiridis K, Treatment of non-small cell lung cancer (NSCLC). J Thorac Dis. 2013;5(SUPPL.4):2–9.
5.Miura Y, Sunaga N. Role of immunotherapy for oncogene-driven non-small cell lung cancer. Cancers (Basel). 2018;10(8):245.
6.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive
review of registration trials and future considerations. J Immunother Cancer. 2018 Dec 23;6(1):8.
7.He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 Pathway in Tumor Immune Microenvironment and Treatment for
Non-Small Cell Lung Cancer. Sci Rep. 2015 Oct 17;5(1):13110.
8.Trotti A, Colevas AD, Setser A, Basch E. Patient-Reported Outcomes and the Evolution of Adverse Event Reporting in Oncology. J Clin Oncol. 2007 Nov 10;25(32):5121–7.
9.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 2017 [cited 2019 Dec 17]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
10.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med. 2015 Jul 9;373(2):123–35.
11.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Nivolumab versus Docetaxel in Advanced Non-squamous Non-small Cell Lung Cancer. 2015 Oct 22;373(17):1627–1639.
12.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N Engl J Med. 2017 Jun 22;376(25):2415–26.
13.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung
cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017 Jan 21;389(10066):255–65.
14.Herbst RS, Baas P, Kim D-W, Felip E, Pírez-Gracia JL, Han J-Y, Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell
lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016 Apr 9;387(10027):1540–50.
15.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2016 Nov 10;375(19):1823–33.
16.Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally
advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label,
controlled, phase 3 trial. Lancet. 2019 May 4;393(10183):1819–30.
17.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;NEJMoa1809697.
18.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med. 2017 Nov 16;377(20):1919–29.
19.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of
novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014 Jan 21;21(1):15–25.
20.Ogawara D, Soda H, Iwasaki K, Suyama T, Taniguchi H, Fukuda Y, Remarkable response of nivolumab-refractory lung cancer to salvage chemotherapy. Thorac Cancer. 2018 Jan;9(1):175–80.
21.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N Engl J Med. 2018 May 31;378(22):2078–92.
22.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N Engl J Med. 2018 Nov 22;379(21):2040–51.
23.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N Engl J Med. 2019 Nov 21;381(21):2020–31.
24.Cappuzzo F, Jotte R, Vynnychenko I, Stroyakovskiy D, Rodriguez DA, Hussein M, IMpower131: Final OS Results of Carboplatin + Nab-Paclitaxel ± Atezolizumab in Advanced
Squamous NSCLC. In: IASLC 2019 World Conference on Lung Cancer hosted by the International
Association for the Study of Lung Cancer. Barcelona; 2019.
25.Jotte RM, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez Abreu D, Hussein MA, IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab
+ carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L
therapy in advanced squamous NSCLC. J Clin Oncol. 2018 Jun 20;36(18_suppl):LBA9000–LBA9000.
26.Cappuzzo F, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, IMpower130: Progression-free survival (PFS) and safety analysis from a randomised
phase III study of carboplatin + nab-paclitaxel (CnP) with or without atezolizumab
(atezo) as first-line (1L) therapy in advanced non-squamous NSCLC. Ann Oncol. 2018 Oct; 29(suppl_8): mdy424.065.
27.Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy
in cancer; lessons learned from clinical trials with melanoma and non-small cell lung
cancer (NSCLC). J Immunother Cancer. 2018 Dec 16;6(1):39.
28.Rizvi NA, Chul Cho B, Reinmuth N, Lee KH, Ahn M-J, Luft A, Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line
treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol. 2018;29(suppl_10):x39–x43.
29.Mok T, Schmid P, Arén O, Arrieta O, Gottfried M, Jazieh AR, 192TiP: NEPTUNE: A global, phase 3 study of durvalumab (MEDI4736) plus tremelimumab
combination therapy versus standard of care (SoC) platinum-based chemotherapy in the
first-line treatment of patients (pts) with advanced or metastatic NSCLC. J Thorac Oncol. 2016 Apr 1;11(4):S140–1.
30.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint
Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018 Jun 10;36(17):1714–68.
31.Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations
from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):1–28.
32.Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically
ill patient. Ann Pharmacother. 2014 Jun 20;48(6):806–10.
33.Pagès C, Gornet JM, Monsel G, Allez M, Bertheau P, Bagot M, Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013 Jun;23(3):227–30.
34.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006 Jan 15;118(2):257–62.
35.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Clinical and biological features associated with epidermal growth factor receptor
gene mutations in lung cancers. J Natl Cancer Inst. 2005 Mar 2;97(5):339–46.
36.Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, The relationship between epidermal growth factor receptor mutations and clinicopathologic
features in non-small cell lung cancers. Clin Cancer Res. 2005 Feb 1;11(3):1167–73.
37.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010 Jun 24;362(25):2380–8.
38.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Erlotinib versus standard chemotherapy as first-line treatment for European patients
with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre,
open-label, randomised phase 3 trial. Lancet Oncol. 2012 Mar;13(3):239–46.
39.Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR
mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre,
open-label, randomised, phase 3 study. Lancet Oncol. 2011 Aug;12(8):735–42.
40.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive
non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE
study. Ann Oncol. 2015 Sep;26(9):1883–9.
41.Wu Y-L, Lee JS, Thongprasert S, Yu C-J, Zhang L, Ladrera G, Intercalated combination of chemotherapy and erlotinib for patients with advanced
stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013 Jul;14(8):777–86.
42.Miller VA, Hirsh V, Cadranel J, Chen Y-M, Park K, Kim S-W, Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung
cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy
(LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012 May;13(5):528– 38.
43.Soria J-C, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, Afatinib versus erlotinib as second-line treatment of patients with advanced squamous
cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase
3 trial. Lancet Oncol. 2015 Aug;16(8):897–907.
44.Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017 Feb 16;376(7):629–640.
45.Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N Engl J Med. 2017 Nov;378(2):113–25.
46.Le T, Gerber DE. ALK alterations and inhibition in lung cancer. Semin Cancer Biol. 2016/09/13. 2017 Feb;42:81–8.
47.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell. 2007 Dec 14;131(6):1190–203.
48.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, Crizotinib versus Chemotherapy in Advanced ALK -Positive Lung Cancer. N Engl J Med. 2013 Jun 20;368(25):2385–94.
49.Solomon BJ, Reisman A, Mok T, Wilner KD, Paolini J, Blackhall F, First-Line Crizotinib versus Chemotherapy in ALK -Positive Lung Cancer. N Engl J Med. 2014 Dec 4;371(23):2167–77.
50.Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung
cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016 Apr;17(4):452–463. doi: http://dx.doi.org/10.1016/S1470-2045(15)00614-2
 .
.
51.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2017 Aug 31;377(9):829–838.
52.Camidge DR, Kim HR, Ahn M-J, Yang JC-H, Han J-Y, Lee J-S, Brigatinib versus Crizotinib in ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2018 Nov 22;379(21):2027–2039.
53.Herbst RS, Davies MJ, Neal JW, Sagorsky S, Gandhi L, Antonia SJ, The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the
treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6(1):1–15.
54.Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin C-C, Soo RA, ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma
Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol. 2019 Jun 1;37(16):1370–9.
55.Shaw AT, Solomon BJ, Chiari R, Riely GJ, Besse B, Soo RA, Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label,
single-arm, phase 1-2 trial. Lancet Oncol. 2019 Dec;20(12):1691–1701
56.Drilon A, Sankhala KK, Liu S V., Cho BC, Blakely C, Chee CE, Abstract CT060: STARTRK-2: A global phase 2, open-label, basket study of entrectinib
in patients with locally advanced or metastatic solid tumors harboring TRK, ROS1,
or ALK gene fusions. In: Clinical Trials. American Association for Cancer Research; 2017. p. CT060–CT060.
57.Grizzi G, Caccese M, Gkountakos A, Carbognin L, Tortora G, Bria E, Expert Review of Molecular Diagnostics Putative predictors of efficacy for immune
checkpoint inhibitors in non-small-cell lung cancer : facing the complexity of the
immune system. Expert Rev Mol Diagn. 2017;17(12):1055–69.
58.Voong KR, Feliciano J, Becker D, Levy B. Beyond PD-L1 testing-emerging biomarkers for immunotherapy in non-small cell lung
cancer. Ann Transl Med. 2017 Sep; 5(18):376.
59.Huang M, Pietanza MC, Samkari A, Pellissier J, Burke T, Chandwani S, Q-TWiST Analysis to Assess Benefit–Risk of Pembrolizumab in Patients with PD-L1–Positive
Advanced or Metastatic Non-small Cell Lung Cancer. Pharmacoeconomics. 2019 Jan 4;37(1):105–16.
60.Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, Cost Effectiveness of Pembrolizumab vs. Standard-of-Care Chemotherapy as First-Line
Treatment for Metastatic NSCLC that Expresses High Levels of PD-L1 in the United States. Pharmacoeconomics. 2017 Aug 15;35(8):831–44.
61.Criss SD, Mooradian MJ, Sheehan DF, Zubiri L, Lumish MA, Gainor JF, Cost-effectiveness and Budgetary Consequence Analysis of Durvalumab Consolidation
Therapy vs No Consolidation Therapy After Chemoradiotherapy in Stage III Non–Small
Cell Lung Cancer in the Context of the US Health Care System. JAMA Oncol. 2019 Mar 1;5(3):358.
62.Lange A, Prenzler A, Frank M, Golpon H, Welte T, von der Schulenburg J-M. A systematic review of the cost-effectiveness of targeted therapies for metastatic
non-small cell lung cancer (NSCLC). BMC Pulm Med. 2014 Dec 4;14(1):192.
63.Ting J, Tien Ho P, Xiang P, Sugay A, Abdel-Sattar M, Wilson L. Cost-Effectiveness and Value of Information of Erlotinib, Afatinib, and Cisplatin-Pemetrexed
for First-Line Treatment of Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer
in the United States. Value Heal. 2015 Sep;18(6):774–82.
64.Lu S, Yu Y, Fu S, Ren H. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell
lung cancer in China. Gangopadhyay N, editor. PLoS One. 2018 Oct 23;13(10):e0205827.
65.Wang S, Peng L, Li J, Zeng X, Ouyang L, Tan C, A Trial-Based Cost-Effectiveness Analysis of Erlotinib Alone versus Platinum-Based
Doublet Chemotherapy as First-Line Therapy for Eastern Asian Nonsquamous Non-Small-Cell
Lung Cancer. PLoS One. 2013;8 (3) (no(e55917).
Sze Wah Samuel Chan, 1 Medical Student, Faculty of Medicine, University of Toronto, Canada
Elliot Smith, 1 Medical Student, Faculty of Medicine, University of Toronto, Canada
Mihnea-Alexandru Găman, Editor
Madeleine Jemima Cox, Student Editor
Correspondence: Sze Wah Samuel Chan, Address: 1 King's College Cir, Toronto, ON M5S 1A8, Canada Email:
schan93@gmail.com
*
Both authors are co-first authors
Cite as: Chan, S, Smith, E. Next Generation of Advanced Non-Small Cell Lung Cancer Therapy: Targeted and Immuno-Therapies. Int J Med Students. 2020 Jan-Apr;8(1):26-32.
Copyright © 2020 Sze Wah Samuel Chan, Elliot Smith
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 8, NUMBER 1, April 2020

