Case Report
Spinal Cord Injury Induced Osteoporosis: Case Report and Current Literature
Abdulai Bangura1, Thomas Shuler2, Lisa Wright3, Anne Lake4
doi: http://dx.doi.org/10.5195/ijms.2021.535
Volume 9, Number 2: 162-166
Received 23 04 2020:
Rev-request 28 10 2020:
Rev-recd 07 04 2021:
Accepted 26 04 2021
ABSTRACT
Background:
Among the various etiologies of osteoporosis, spinal cord injury has a drastic progression
of the disease, causing weekly bone loss. There is no definitive treatment for the
prevention of osteoporosis in these individuals. This review illustrates the recent
findings on the pathophysiology, treatment, and management of spinal cord injury-induced
osteoporosis. Furthermore, we cover a case of a male patient who experienced severe
bone loss after a spinal cord injury at the age of 21 years.
The Case:
We have a 57-year-old man with a history of AIS grade A spinal cord injury, level
T11 with rod fixation from a motorcycle collision at age 21. His fracture history
following the injury includes tibia, femur, and vertebral fractures. Bone mineral
density imaging revealed notable T-scores ranging from −3.1 to −3.4 at the hip and
femurs. Treatment plan consisted of teriparatide, dietary supplements, and physical
therapy. Biomarkers from baseline to post one month of treatment revealed the following:
procollagen type 1 N-terminal propeptide from 38 mcg/L to 70 mcg/L and C-terminal
telopeptide from 209 pg/mL to 88 pg/mL, representing an increased bone formation and
decreased bone resorption, respectively. After two years, bone mineral density T-scores
improved to −2.7 on the left and the patient was capable of standing for the first
time with the assistance of a standing frame.
Conclusion:
Our case exemplified the progression of the disease and treatment options. A basis
for the derivation of future innovative therapies has been covered. Favorable treatments
and management are described in the review.
Keywords:
Spinal Cord Injuries;
Osteoporosis;
Teriparatide;
Bone Density;
SOST protein;
human (Source: MeSH-NLM).
Highlights:
- The most recent findings on the pathophysiology, treatment, and management of spinal
cord injury induced osteoporosis.
- A basis for the derivation of future innovative therapies for spinal cord injury induced
osteoporosis.
- Favorable treatments and management for best prognosis in spinal cord injury induced
osteoporosis.
Introduction
Among the various etiologies of osteoporosis, spinal cord injury (SCI) has a drastic
progression of the disease, causing weekly bone loss. This is due to a multifactorial
and unfavorable set of consequences involving bone metabolism.1 Following bone peak mass at 30 years of age, men and women lose bone mineral density
(BMD) at a rate of 0.3% and 0.5% per year, respectively. Post-menopausal women lose
BMD at a rate of 2% per year.2 However, individuals with SCI lose 1% of BMD per week.1 Due to the significant amount of bone loss leading to osteoporotic fractures, individuals
with SCI are at an increased risk for comorbidities including osteomyelitis, skin
pressure ulcers from bracing and bedrest, and hypertensive crisis from autonomic dysreflexia.3,4 The National Spinal Cord Injury Statistical Center (NSCISC) reports an incidence
of 17,810 new SCI cases in the United States each year with a current prevalence that
could reach 368,000 people. According to the NSCISC data sheets, both the incidence
and prevalence have increased over the last couple years with the most common cause
being motor vehicle accidents. Additional common causes are acts of violence (primarily
gunshot wounds) and sports/recreational injuries.5,6 At this time, there is no definitive treatment for the prevention of osteoporosis
in these individuals. We hope to establish the current pathophysiology to provide
a basis for future innovative therapies.
People with SCI are immediately challenged by the consequences of mechanical unloading,
neural denervation with subsequent vascular dysregulation, and biomarker abnormalities.
All of which contribute to either increased bone resorption, decreased bone formation,
or a combination of two. Mechanical unloading is noteworthy as it leads to a cascade
of events that is expected to have the strongest association with bone loss.3
In both human and animal studies, a decrease of mechanical loading on bone has been
found to have a significant association with an increase in sclerostin protein synthesis
and vice-versa.7,8 Sclerostin is encoded by the sclerostin gene (SOST) and it is expressed by many tissues,
but primarily by osteocytes. Most recent literature has recognized sclerostin as the
principal mediator of SCI osteoporosis.3 Sclerostin inhibits the Wnt/β-catenin pathway, which is a vital component of bone
formation.9 Furthermore, sclerostin increases the expression of receptor activator of nuclear
factor kappa-β ligand (RANKL) and decreases the expression of receptor activity of
osteoprotegerin (OPG) which ultimately increases bone resorption.10
Due to sclerostin's strong correlation with mechanical unloading, it is a great contributor
to SCI osteoporosis. Sclerostin demonstrates an inverse relationship with BMD within
the first 5 years following SCI, with sclerostin levels increasing as BMD decreases.
After 5 years, the relationship reverses into a positive relationship with sclerostin
levels now decreasing as BMD levels continue to decrease.11 One study sampled men with chronic SCI (2+ years post injury) and as the number of
years following the SCI increased, the levels of sclerostin and BMD decreased together.
It is important to note that the duration of injury for the subjects ranged from 4.1
to 42.6 years. Their findings suggested that circulating sclerostin levels in chronic
SCI is a potential indicator of osteoporosis severity.12
Although mechanical unloading appears to be the point of attention, other factors
impact SCI osteoporosis. As expected, there would be neural damage which reduces bone
function. Sympathetic stimulation contributes to bone maintenance and it has been
found that sympathetic denervation of bone in animal models revealed increased bone
resorption and decreased bone mineralization. Furthermore, sympathetic denervation
causes subsequent vascular dysregulation. The impairment of vascular regulation allows
increased capillary and venous blood pooling which leads to increased intraluminal
pressure. A potential consequence of local blood pooling is osteoclast formation.4 For these reasons, sympathetic denervation and subsequent vascular dysregulation
are potential contributors to osteoporosis in individuals with SCI.
In addition to mechanical unloading and sympathetic denervation, biomarkers including
vitamin D, parathyroid hormone (PTH), and fat, contribute to osteoporosis in individuals
with SCI. Although the prevalence of vitamin D deficiency is high among the general
population, people with SCI are still at an increased risk for vitamin D insufficiency
or deficiency.13 In regards to PTH, the high activity of bone resorption following SCI induces hypercalcemia
leading to the suppression of PTH synthesis.14 PTH has been found to suppress sclerostin levels in human and animal studies.15,16 Therefore, a decrease in PTH can subsequently lead to further bone loss by increasing
sclerostin levels. Fat has also been found to affect bone maintenance in SCI individuals.
Multiple studies have revealed that people with SCI have a greater percentage of body
fat in comparison with matched age and sex controls, demonstrating a greater risk
of obesity in people with SCI than in the general population.3
It is established that fat has an osteoprotective effect on bone by way of increased
mechanical loading which induces bone formation.17 However, with SCI, muscle paralysis prevents mechanical loading and may disrupt this
process. In addition, fat releases leptin which mainly regulates appetite and energy
expenditure in the hypothalamus.18 Leptin also has additional properties including bone formation regulation. Leptin
can provide sympathetic inhibition to osteoblasts and suppress bone formation by way
of beta-2-adrenergic receptors on the osteoblast cell surface.3 Leptin levels have been shown to be elevated in individuals with SCI in comparison
with the general population.19 Adiponectin is a hormone that is produced by adipocytes or fat cells. In both human
and animal studies, increased levels of adiponectin have been associated with increased
bone loss.3 One study revealed that adiponectin has an inverse relationship with BMD in individuals
with chronic SCI. The same study revealed that when these individuals participated
in walking activities, the inverse relationship was no longer found.20 However, research of the relationship between adiponectin and bone loss specifically
in the SCI population is currently ongoing. Therefore, a deficiency of vitamin D or
PTH, and an accumulation of fat could all potentially contribute to osteoporosis in
individuals with SCI.
We present a case of a male patient who experienced severe bone loss after a SCI at
the age of 21 years and review the literature to discuss treatment options.
The Case
History
A 57-year-old man with a history of level T11, AIS grade A SCI with rod fixation from
a motorcycle accident at age 21 was referred to our fracture liaison and bone health
clinic for a bone health evaluation. The patient himself provided consent for his
information to be included in publications. He is 5'10” with a body mass index (BMI)
of 23.8. He has a 7.5 pack-years smoking history with cessation of smoking in 2015.
His fracture history following the SCI included right tibia fracture, right femur
fracture, and left femur fracture all of which were associated with the mechanical
attempts of standing and therapy. There is no record of his treatment plan or laboratory
results prior to his first visit at our bone health clinic.
Investigation
Physical examination was consistent with a T11 level paraplegia with anesthesia at
the T12 dermatome and motor examination with flaccid lower extremity paralysis, and
2+ distal pulses. The left lower extremity had a slight knee contracture of 5 to 10
degrees. Bone density imaging referenced severe osteoporosis in the total hip and
femoral neck bilaterally (Table 1). BMD imaging revealed the following notable T-scores: right hip and right femoral
neck [T-score −3.1], left hip and left femoral neck [T-score −3.4]. However, it is
important to note that optimal leg positioning for BMD imaging was not achieved due
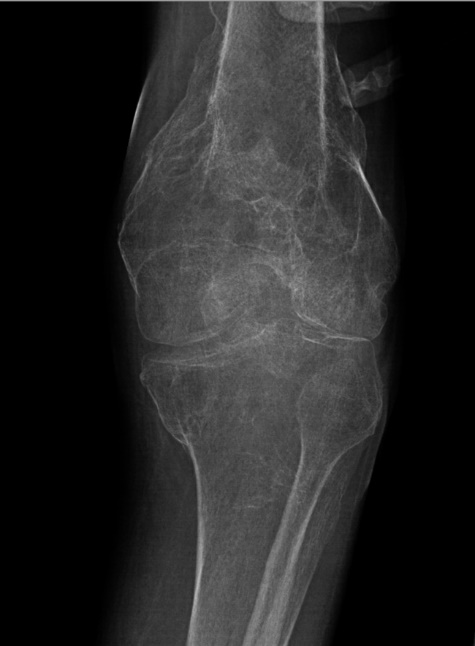
to the patient's limitations. Furthermore, radiological imaging represented diffuse
bony demineralization of the left femur (Figure 1). Laboratory orders were ascertained for a baseline which included bone biomarkers
for comparing with post treatment evaluations (Table 2).
Table 1.
Bone density imaging referenced severe osteoporosis in the total hip and femoral neck
bilaterally.
| Component |
Value |
Comment |
| BMD Spine (L1-L4) |
1.282 |
Could only scan L3-L4 due to hardware in L1-L2 |
| T-Score Spine (L1-L4) |
0.4 |
|
| Z-Score Spine (L1-L4) |
0.7 |
|
| BMD Right Hip |
0.671 |
|
| T-Score Right Hip |
−3.1 |
Osteoporosis |
| Z-Score Right Hip |
−2.2 |
|
| BMD Left Hip |
0.622 |
|
| T-Score Left Hip |
−3.4 |
Osteoporosis |
| Z-Score Left Hip |
−2.6 |
|
| BMD Mean Hip |
0.674 |
Unable to position legs optimally for scanning |
| T-Score Mean Hip |
−3.3 |
osteoporosis |
| Z-Score Mean Hip |
−2.4 |
|
| BMD Right Femoral Neck |
0.671 |
|
| T-Score Right Femoral Neck |
−3.1 |
osteoporosis |
| Z-Score Right Femoral Neck |
−2.2 |
|
| BMD Left Femoral Neck |
0.622 |
|
| T-Score Left Femoral Neck |
−3.4 |
osteoporosis |
| Z-Score Left Femoral Neck |
−2.6 |
|
Table 2.
Baseline and post-therapy biomarkers.
| Lab |
Reference range |
Baseline |
1 month |
| P1NP |
30–110 mcg/L |
38 mcg/L |
70 mcg/L |
| Vitamin D |
30–100 ng/mL |
26 ng/mL |
50 ng/mL |
| Alk Phos Bone |
7.6–14.9 mcg/L |
12.1 mcg/L |
|
| CTX |
87–345 pg/mL |
209 pg/mL |
88 pg/mL |
| PTH |
18.4–88.0 pg/mL |
52.8 pg/mL |
|
| Phosphorous |
2.5–4.6 mg/dL |
3.3 mg/dL |
|
| Calcium |
8.5–10.7 mg/dL |
9.8 mg/dL |
10.4 mg/dL |
| Creatinine |
0.5–1.4 mg/dL |
0.46 mg/dL |
<0.38 mg/dL |
| Ionized Calcium |
1.13–1.32 mmol/L |
|
1.19 mmol/L |
Figure 1
Left knee x-ray (oblique, externally rotated). Diffuse bony demineralization reduces
sensitivity of radiography for acute fracture. There is corticated deformity of the
distal femur and proximal tibia from old, healed fractures. No clear evidence of a
superimposed acute fracture.
Management
We considered the patient's past history, imaging, and laboratory results to align
the following treatment plan: teriparatide [rDNAorigin] injection once daily, vitamin
D2 (50,000 iu's) once weekly for 8 weeks, vitamin D3 (2000 iu's) once daily, vitamin
K2, and calcium citrate 600 mg once daily. Additional supplements included magnesium
citrate and creatine. Treatment medication was chosen based upon the goal of promoting
bone formation and synergistically aligning the patient to a standing frame. Treatment
decisions were made based upon current available literature and shared decision making
between the patient. Outpatient physical therapy was prescribed to promote resistance
upper body training to help promote bone growth in addition to his use of teriparatide.
The patient was also followed by physical medicine and rehabilitation with which a
standing frame was attempted but not achieved. After one year of treatment, care was
transferred to another fracture liaison and bone health clinic. At the new site, the
patient continued with teriparatide for an additional year, which was then discontinued
due to its black box warning. There is a theoretical risk of osteosarcoma when medicating
with teriparatide for more than 2 years. Vitamin D3 supplementation was also continued,
but at a greater dose, 3000 iu's once daily. While promoting bone formation with biologic
measures, the mechanical goal of aligning the patient to a standing frame remained
the same.
Outcome
Notable biomarkers from baseline to post one month of treatment revealed the following:
procollagen-1 N-terminal peptide (P1NP) from 38 mcg/L to 70 mcg/L and C-terminal telopeptide
(CTX) 209 pg/mL to 88 pg/mL, representing an increased bone formation and decreased
bone resorption, respectively. The patient's symptoms regarding immobility and fracture
risk remained the same at that time. After two years of treatment, there was improvement
in BMD represented at the left femoral neck [T-score −2.7] and left total hip [T-score
−2.7] which both improved from baseline [T-score −3.4] (Table 1). Furthermore, the patient was capable of utilizing a standing frame and stood for
the first time since before his injury 38 years prior.
Discussion
Our case revealed improvement in osteoporosis labs and physical symptoms during a
two-year course of treatment. Once labs and bone density tests have leveled, we would
expect them to be at a steady state barring overall health change. We believe the
improvement seen in our patient's BMD was supported by prescribing teriparatide, supplementing
vitamin D, and utilizing a standing frame. During the time of management, we were
not informed of the International Society for Clinical Densitometry (ISCD) guidelines
and did not utilize them. This patient could have also been a good candidate to consider
the latest technology VirtuOst Stress Test due to the difficulty in patient positioning
from his previous bone density imaging. VirtuOst Stress Test is a Food Drug Administration
(FDA) cleared virtual stress test that assesses BMD, bone strength, and fracture risk.
Bone density does not fully assess bone strength and quality. Factors such as diet,
smoking status, alcohol use, are also important associated factors to evaluate in
a patient with osteoporosis. In this patient's case, we focused on a weight training
program aligned with a physical therapist to promote body strength which over time
had weakened in addition to providing mechanical load to the skeleton.
Due to the black box warning on teriparatide, the patient's medication was switched
to denosumab, a monoclonal antibody against RANKL. The decision to transition to denosumab
was extrapolated from the DATA-switch study.21 There has since been an update to teriparatide's label, removing the black box warning.
The decision to resume teriparatide after two years is determined by clinical decision
making and risk-benefit considerations. Given the recent update regarding teriparatide,
it is reasonable to evaluate the patient one year after denosumab to determine whether
to consider a future return to an anabolic therapy such as teriparatide. It is important
to accrue bone mass over time by structuring a sequence of pharmacologic therapy.
If our patient's future progression becomes similar to previous studied cases, then
we should expect a cessation in lab and bone density test improvement and minimal
or no improvement of symptoms. For these reasons, it is important to illustrate the
most recent findings of the pathophysiology, treatment, and management of SCI osteoporosis
to reference optimal care and provide a basis for the derivation of future innovative
therapies.
Although the pathophysiology of SCI osteoporosis has been distinctly outlined, the
treatments' efficacy remains limited.22
Current Treatments
An effective long-term treatment for SCI osteoporosis has not been established. Current
treatment options include pharmacological and physical therapy interventions. Although
there are no interventions which prevent or reverse SCI osteoporosis, bisphosphonates,
a group of antiresorptive drugs, are the most common pharmacological treatment for
bone loss prevention in these individuals. Unfortunately, bisphosphonates have mostly
been shown to be effective within the first year post SCI.4 Studies have shown a 16.4% to 19.7% reduction in bone loss at the femoral neck and
approximately 21% percent reduction in bone loss at the total hip when treating SCI
osteoporosis with bisphosphonates within the first year.23 However, a single study revealed that a two year course of bisphosphonates following
SCI reduces the risk of fracture for two years, but revealed no evidence of bone loss
prevention following one year.3 Bisphosphonates aid in the prevention of acute bone loss following SCI, but have
no effect on bone formation. Therefore, the effect of bisphosphonates is not substantial.
This dilemma has encouraged further investigation for more desirable pharmacological
treatments.
Teriparatide (TPDT), a recombinant human parathyroid hormone has recently gained attention
as the optimal pharmacological treatment. TPDT is one of the few approved anabolic
pharmacological treatments for osteoporosis and can be effective up until 24 months.24 It has also shown efficacy in treatment of SCI osteoporosis demonstrating a 4.8%
to 5.5% increase in spinal BMD from baseline to 12 months. Furthermore, TPDT revealed
a 7.1% to 14.4% increase in spinal BMD from baseline to 24 months.25 Along with these current drug therapies, denosumab, a monoclonal antibody against
RANKL has been shown to increase BMD in individuals with SCI induced osteoporosis
as well.26
As for physical therapy interventions, weight-bearing exercises, functional electrical
stimulation (FES), and whole-body vibration (WBV) have been used to improve osteoporosis
by increasing BMD.3,4 As we discussed earlier, sclerostin levels decrease with mechanical loading. Therefore,
an increase of bone formation should be expected to follow weight-bearing exercises.
Mechanical loading in SCI is a considerable challenge given the immobile state of
the person. However, this challenge has been approached with FES exercises. FES treatment
achieves mechanical loading by allowing electrodes to stimulate paralyzed muscles
and facilitate muscle contraction. FES exercises have not been proven to provide long-term
efficacy.
Whole body vibration (WBV) therapy also can achieve mechanical loading via mechanical
vibration and is currently a potential treatment for bone formation in SCI. In fact,
both human and animal studies have reported neurological function recovery with SCIs
after WBV therapy.27,28 The efficacy of WBV therapy on osteoporosis in SCI has not been thoroughly evaluated.
However, one study was able to report an increase of percentage in BMD only at the
knee after 12 months of WBV therapy.25
Potential Treatments
Additional potential treatments for SCI induced osteoporosis include romosozumab,
abaloparatide, activin receptor blockers, and cathepsin-K inhibitors. Romosozumab
(ROMO) is a new anti-sclerostin drug that has revealed a significantly greater improvement
in BMD and reduced fracture risk in comparison with teriparatide treatment in post-menopausal
women with osteoporosis.29 Although ROMO has not yet been approved for men due to serious cardiovascular side
effect risks, it has shown promising results of BMD improvement in its phase III clinical
trial.30 There is a lack of research on the effects of ROMO administration on the bone metabolism
of the SCI population.
Abaloparatide is a bone forming agent used to treat post-menopausal osteoporosis in
women who have failed antiresorptive therapy or have a high risk of fracture. Abaloparatide
usage reduces the risk of osteoporotic fractures and the prevalence of hypercalcemia
in comparison with teriparatide. Furthermore, it is more cost effective than teriparatide.31 Again, there is a lack of research on the effects of abaloparatide administration
on the bone metabolism of the SCI population. Not to mention, both romosozumab and
abaloparatide have not yet been approved for males. Although romosozumab and abaloparatide
have not proved their efficiency in SCI osteoporosis, they both remain potential pharmacological
treatments.
Additional but less effective potential treatments currently being reviewed are type
II activin receptor (ActRIIA) blockers and cathepsin-K inhibitors. Up to date, the
efficacy of ActRIIA blockade has not been reviewed in people with SCI. Cathepsin-K
is a protease involved in bone catabolism and research unveiled early bone loss prevention
in post-menopausal women with cathepsin-K inhibitors.3 However, cathepsin-K inhibitors have been found to increase the risk of stroke which
led to the termination of its development, specifically odanacatib.32 Therefore, ActRIIA blockers and cathepsin-K inhibitors remain potential pharmacological
treatments for SCI osteoporosis as well.
Management
As for management, serial dual-energy X-ray absorptiometry (DXA) scans are utilized
concurrently with bone biomarker monitoring and correction.25 Up to now, the identification of clinical improvement via DXA scans has been limited
due to the absence of an established guideline for SCI osteoporosis. Fortunately,
the ISCD recently developed a task force to perform a multi-study review of the DXA
scan's role during various aspects of SCI osteoporosis management. This review allowed
the ISCD to create their official position statement on BMD testing in SCI. The official
position statement reports the following verbatim:
“1. All adults with spinal cord injury resulting in permanent motor or sensory dysfunction
should have a DXA scan of the total hip, proximal tibia, and distal femur as soon
as medically stable.
2. In adults with SCI, total hip, distal femur and proximal tibia bone density should
be used to diagnose osteoporosis, predict lower extremity fracture risk, and monitor
response to therapy where normative data is available.
3. Serial DXA assessment of treatment effectiveness among individuals with SCI should
include evaluation at the total hip, distal femur, and proximal tibia, following a
minimum of 12 months of therapy at 1- to 2-year intervals. Segmental analysis of total
hip, distal femur and proximal tibia sub-regions from a whole-body scan should not
be used for monitoring treatment.
4. There is no established threshold BMD value below which weight-bearing activities
are absolutely contraindicated. BMD and clinical risk factors should be used to assess
fracture risk prior to engaging in weight-bearing activities.”
It is notable that the ISCD added the necessity of the person having sufficient turning
radius for a manual or power wheelchair during the scan and that the chair must be
equipped with a lift. Furthermore, focused areas containing artifacts should be recognized
and should not be used for diagnosis, fracture risk assessment, or monitoring response
to therapy. Some examples of artifacts include hardware, deformity, heterotopic ossification,
contracture or movement (spasticity), or leg bag artifacts which prevent optimal position
for scanning or limit the accuracy of the analysis.33
The CTX is a marker of bone resorption (degradation) and the P1NP is a marker for
bone formation.34 The biomarkers are used as a tool to help identify the appropriate recommendations
for treatment along with other factors in the patient's history such as previous osteoporosis
treatment plan, comorbid conditions, among other factors. In the literature, in treatment
of naïve patients, an increase in P1NP was a predictor of BMD at 12 months.35 There is no comparator to a median or expected ranges in this case but rather an
improvement from baseline.
Conclusion
The findings in this case provide hope for bone health and strength in SCI patients
as they continue to age with their disability. Newer anabolics, such as abaloparatide
and romosozumab, have shown greater improvement than previous treatments in osteoporosis.
We believe using optimal pharmacological agents, mechanical loading of the skeleton,
and the ISCD guidelines will allow the best patient prognosis for the general population
of SCI patients. The pathophysiology of spinal cord injury induced osteoporosis has
been distinctly outlined. Our case exemplified progress that can be made with aggressive
mechanical and biologic treatment. A basis for the derivation of future innovative
therapies has been covered. Favorable treatments and management are referenced for
best patient prognosis.
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions
Conceptualization and Visualization: TS. Investigation: LW. Methodology, Supervision
and Writing – Original Draft Preparation: AB. Writing – Review & Editing: TS and LW.
Acknowledgments
None
References
1. Bauman WA, Cardozo CP. Osteoporosis in Individuals with Spinal Cord Injury. PM&R. 2014 Aug 27;7(2):188–201.
2. Dobbs NB, Buckwalter J, Saltzman C. Osteoporosis: The Increasing Role of the Orthopaedist. Iowa Orthop J. 1999; 19:43–52.
3. Battaglino RA, Lazzari AA, Garshick E, Morse LR. Spinal Cord Injury-Induced Osteoporosis: Pathogenesis and Emerging Therapies. Curr Osteoporos Rep. 2012 Dec;10(4):278–85.
4. Tan CO. Spinal Cord Injury and Osteoporosis: Causes, Mechanisms, and Rehabilitation Strategies. Int J Phys Med Rehabil. 2013;1(4):127.
5. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figueres at a Glance. Available from https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202018.pdf. Last updated 2021; cited Jun 22, 2020.
6. National Spinal Cord Injury Statistical Center. Spinal Cord Injury Facts and Figures at a Glance. Available from: https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf. Last updated 2021; cited Jun 22, 2020.
7. Li X, Ominsky MS, Niu Q-T, Sun N, Daugherty B, Dagostin D, et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation
and Bone Strength. J Bone Miner Res. 2008 Jun 11;23(6):860–9.
8. Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, et al. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012 Feb;27(2):352–9.
9. Wu J, Ma L, Wu L, Jin Q. Wnt-β-catenin signaling pathway inhibition by sclerostin may protect against degradation
in healthy but not osteoarthritic cartilage. Mol Med Rep. 2017 May;15(5):2423–32.
10. Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin Stimulates Osteocyte Support of Osteoclast Activity by a RANKL-Dependent
Pathway. PLoS ONE. 2011 Oct;6(10):E25900.
11. Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone. 2012 Sept;51(3):600–5.
12. Morse LR, Sudhakar S, Lazzari AA, Tun C, Garshick E, Zafonte R, et al. Sclerostin: a candidate biomarker of SCI-induced osteoporosis. Osteoporos Int. 2013 Mar;24(3):961–8.
13. Lamarche J, Mailhot G. Vitamin D and spinal cord injury: should we care? Spinal Cord. 2016 Dec;54(12):1060–75.
14. Mechanick JL, Pomerantz F, Flanagan S, Stein A, Gordon WA, Ragnarsson KT. Parathyroid hormone suppression in spinal cord injury patients is associated with
the degree of neurologic impairment and not the level of injury. Arch Phys Med Rehabil. 1997 Jul;78(7):692–6.
15. Drake M, Srinivasan B, Mödder U, Peterson J, McCready L, Riggs B, et al. Effects of Parathyroid Hormone Treatment on Circulating Sclerostin Levels in Postmenopausal
Women. J Clin Endocrinol Metab. 2010 Nov;95(11):5056–62.
16. Yu EW, Kumbhani R, Siwila-Sackman E, Leder BZ. Acute Decline in Serum Sclerostin in Response to PTH Infusion in Healthy Men. J Clin Endorcinol Metab. 2011 Nov;96(11):E1848–51.
17. Savvidis C, Tournis S, Dede AD. Obesity and bone metabolism. Hormones. 2018 Apr;17(2):205–17.
18. Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013 Jun;7(2):207–22.
19. Jeon JY, Steadward RD, Wheeler GD, Bell G, Mccargar L, Harber V. Intact Sympathetic Nervous System Is Required for Leptin Effects on Resting Metabolic
Rate in People with Spinal Cord Injury. J Clin Endocrinol Metab. 2003 Jan;88(1):402–7.
20. Doherty AL, Battaglino RA, Donovan J, Gagnon D, Lazzari AA, Garshick E, et al. Adiponectin Is a Candidate Biomarker of Lower Extremity Bone Density in Men With Chronic
Spinal Cord Injury. J Bone Miner Res. 2014 Jan;29(1):251–9.
21. Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in post-menopausal osteoporosis (the DATA-switch
study): extension of a randomized control trial. Lancet. 2015 Sep 19;386(9999):1147–1155
22. Morse L. Osteoporosis prophylaxis in acute SCI. Spinal Cord Ser Cases. 2019 Apr;5(1).
23. Goenka S, Sethi S, Pandey N, Joshi M, Jindal Rajeswari. Effect of Early Treatment with Zoledronic Acid on Prevention of Bone Loss in Patients
with Acute Spinal Cord Injury: A Randomized Controlled Trial. Spinal Cord. 2018 Sept 26; 56:1207–1211.
24. Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int. 2016 Aug;27(8):2395–410.
25. Edwards WB, Simonian N, Haider IT, Anschel AS, Chen D, Gordon KE, et al. Effects of Teriparatide and Vibration on Bone Mass and Bone Strength in People with
Bone Loss and Spinal Cord Injury: A Randomized, Controlled Trial. J Bone Miner Res. 2018 Oct;33(10):1729–40.
26. StatPearls Publishing. Osteoporosis in Spinal Cord Injuries. Available from https://www.ncbi.nlm.nih.gov/books/NBK526109/. Last updated Dec 2, 2020; cited Apr 23, 2020.
27. Wirth F, Schempf G, Stein G, Wellmann K, Manthou M, Scholl C, et al. Whole-Body Vibration Improves Functional Recovery in Spinal Cord Injured Rats. J Neurotrauma. 2013 Mar 15;30(6):453–68.
28. Ness LL, Field-Fote EC. Whole-body vibration improves walking function in individuals with spinal cord injury:
A pilot study. Gait Posture. 2009 Nov;30(4):436–40.
29. Mcclung MR. Romosozumab for the treatment of osteoporosis. Osteoporos Sarcopenia. 2018 Mar;4(1):11–5.
30. Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, et al. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of
Romosozumab in Men With Osteoporosis. J Clin Endocrinol Metab. 2018 Sept 1;103(9):3183–93.
31. Merlotti D, Falchetti A, Chiodini I, Gennari L. Efficacy and safety of abaloparatide for the treatment of post-menopausal osteoporosis. Expert Opin on Pharmacother. 2019 Mar 11;20(7):805–11.
32. McClung M, O'Donoghue M, Papapoulos S, Bone H, Langdahl B, Saag K. Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre,
randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol. 2019 Dec 1;7(12):899–911.
33. Morse LR, Biering-Soerensen F, Carbone LD, Cervinka T, Cirnigliaro CM, Johnston TE, et al. Bone Mineral Density Testing in Spinal Cord Injury: 2019 ISCD Official Position. J Clinical Densitom. 2019 Oct–Dec;22(4):554–66.
34. Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone Turnover Markers: Emerging Tool in the Management of Osteoporosis. Indian J Endocrinol Metab. 2016 Nov–Dec; 20(6):846–853.
35. Tsujimoto M, Chen P, Miyauchi A, Sowa H, Krege JH. PINP as and Aid for Monitoring Patients Treated with Teriparatide. Bone. 2011 Apr 1; 48(4): 798–803.
Abdulai Bangura, 1 MS. Trinity School of Medicine, Department of Research, Ratho Mill, Saint Vincent
and the Grenadines, United States
Thomas Shuler, 2 MD. Carilion Clinic, Department of Orthopaedics, Roanoke, Virginia, United States
Lisa Wright, 3 DNP. Carilion Clinic, Department of Orthopaedics, Roanoke, Virginia, United States
Anne Lake, 4 DNP. Wake Forest Baptist Health, Department of Orthopaedics, Winston-Salem, North Carolina,
United States
About the Author: Abdulai Bangura is currently a third-year medical student of Trinity School of Medicine,
Ratho Mill, St. Vincent and the Grenadines of a 4-year program. He is also one of
the five fellows selected for the R Adams Cowley Shock Trauma Center (STC) Orthopaedic
Traumatology Research Fellowship for the 2020-2021 year.
Correspondence: Abdulai Bangura, Address: Trinity School of Medicine, Department of Research, Ratho
Mill, Saint Vincent and the Grenadines 925 Woodstock Road, Suite 200, United States.
Email: abdulai.bangura.21@trinitysom.net
Editor: Francisco J. Bonilla-Escobar
Student Editors: David Ben-Nun
Student Editors: Thanthima Suwanthawornkul
Student Editors: Nikoleta Tellios
Copyeditor: Leah Komer
Proofreader: Nikoleta Tellios
Layout Editor: Fatma Monib
Cite as:
Bangura A, Shuler T, Wright L, Lake A. Spinal Cord Injury Induced Osteoporosis: Case
Report and Current Literature. Int J Med Students. 2021 May-Jun;9(2):162-6.
Copyright © 2021 Abdulai Bangura, Thomas Shuler, Lisa Wright, Anne Lake
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 9, NUMBER 2, May-June 2021
