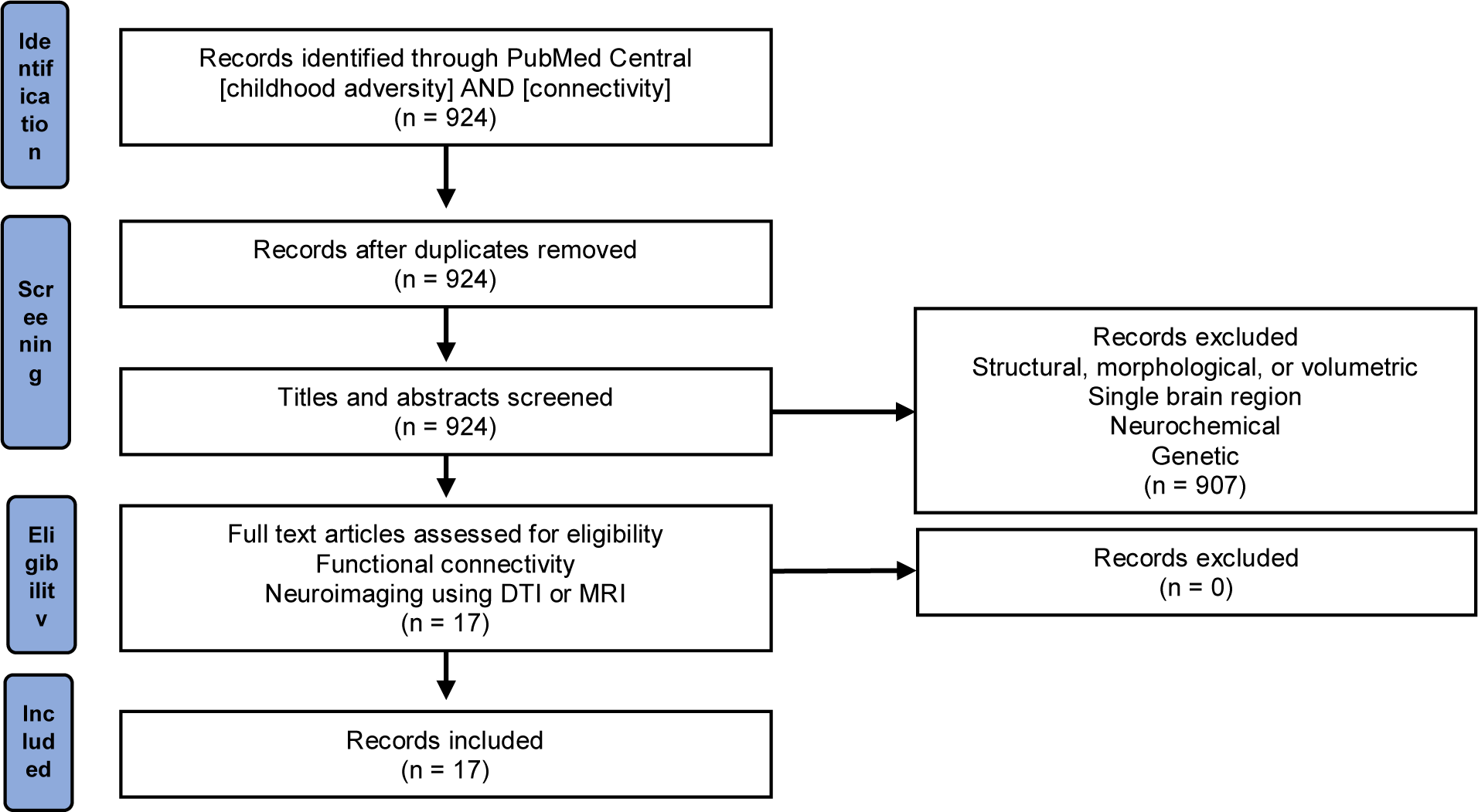
PRISMA Flow Diagram of Literature Search
Alexander L. Shand1
doi: http://dx.doi.org/10.5195/ijms.2021.608
Volume 9, Number 1: 43-51
Received 15 06 2020: Rev-request 05 07 2020: Rev-request 24 11 2020: Rev-request 13 03 2021: Rev-recd 08 07 2020: Rev-recd 30 12 2020: Rev-recd 14 04 2021: Accepted 14 04 2021
ABSTRACT
Children who experience adversity have an increased risk for psychiatric disorders. However, little is known about the exact alterations that occur in the neural circuitry and how that information may help lead to early diagnosis or preventive medicine. Research has shown that there are specific changes in neurological functional connectivity in the brain associated with childhood adversity. This review examined seventeen studies that investigated the correlation between changes in brain connectivity and specific psychiatric disorders. Specifically, it reviews articles that used imaging techniques to directly visualize functional connectivity changes in the brains of children exposed to adversity. Major findings to be discussed in more detail and in different disease states include stronger connectivity from the hippocampus, ventral striatum, amygdala, and in the medial lemniscus. Decreased connectivity strength was found in all the major projection, association, and commissural fiber pathways. Understanding these changes may help with preventive medicine by ensuring that clinicians monitor patients with more severe history of adversity who are therefore at higher risk for developing a psychiatric disorder. This paper will also address potential recommendations that could be implemented in the future as research offers more conclusive evidence. Research is now beginning to address the questions of whether these changes can be attenuated, either during childhood or as adults.
Keywords: Adverse Childhood Experiences; Functional Neuroimaging; Neural Pathways; Psychiatry; Psychopathology; Surveys and Questionnaires (Source: MeSH-NLM).
There is currently no consensus among experts about how many children experience adversity, but estimates for the prevalence of childhood adversity (CA) range from twenty-five percent1 to eighty percent.2 These prevalences are dependent on the cohort examined and there are many more estimates in between these extremes.3–5 Of adolescents in the United States, approximately twenty-percent are affected by mental illness each year.6 There is a link between exposure to childhood adversity and development of mental health disorders,7 however quantitative values remain unknown due to other factors such as heritability.8 It should be noted that while there is a definite association,7 it cannot yet be confidently stated that that there is a causal relationship.
Within the past twenty-five years, research has demonstrated definitive links to specific long-term effects caused by CA.7,9–12 The Adverse Childhood Experiences (ACE) survey is the study that first established links between CA and medical disease such as ischemic heart disease, chronic lung disease, cancer, liver disease.9 Other medical diseases associated with CA include diabetes,13 cardiovascular disease,14 stroke, obesity, chronic obstructive lung disease, autoimmune disease, sexually transmitted disease, and reduced life expectancy.15
In addition to links between CA and medical disease, it is also well established that adversity in children is associated with increased risk for psychiatric disorders.7 The most common psychiatric issues that develop as a result of CA are depression, bipolar disorder, anxiety, eating disorders, suicidal ideation, conduct disorders, and substance use disorders. Of note, for each of these negative consequences there is a dose-dependent relationship between CA exposure and likelihood of development.7
Research then shifted to examine how adversity affects structure, volume, and morphology of the brain, and again many significant and consistent correlations were found; most regions in the brain had decreased volumes. CA exposure causes decreased volumes of the hippocampus, dorsolateral prefrontal cortex, medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, and cerebellum. 10,11 There is conflicting evidence regarding the amygdala and corpus callosum, with some studies identifying increased volumes, but others showing no significant change.10
Moving forward, work then began to look at activity within individual regions of the brain, without yet focusing on connections between regions.12 In people with a history of CA, regions that are hyperactive in response to emotional stimuli include the amygdala, superior temporal gyrus, parahippocampal gyrus, and insula.12
There is substantial evidence that childhood adversity affects neurodevelopment and susceptibility to medical disease and psychopathology. However, there is not much evidence demonstrating specific inter-regional connectivity changes in the neurological circuits and the precise mechanisms of how they contribute to the development of mental health disease. These brain changes are the focus of much of the current research, and the focus of this paper which will review the specific changes in functional connectivity and signaling between regions of the brain in children exposed to adversity. It will also look at the level of the strength of the connectivity between regions and will review the evidence linking these changes to mental illness. Finally, it will discuss how this knowledge could contribute to preventive medicine.
Studies were selected by searching in PubMed Central. The first search criteria was [adverse childhood experiences] AND [MRI] which yielded 61 results. To expand the scope, the second search criteria was [childhood adversity] AND [MRI] which yielded 1068 results. These results were too vague and diverse. To limit the scope, we focus on only those studies that related to functional connectivity. Thus, the third search criteria was [childhood adversity] AND [connectivity] which yielded 924 results. The search was not restricted by any means. Using date filters, we found that from January 1, 2000 – December 31, 2008 only 6 articles were published that met the search criteria. From January 1, 2009 – December 31, 2014 an additional 211 articles were published matching the criteria. Then from January 1, 2015 – February 28, 2020 and additional 730 articles were published. This rapid and robust increase in results matching the search terms implies a shift towards studies relating to whole brain neural mapping and functional connectivity. This rising field of research is where we decided to focus this review. We tried to narrow the scope of this paper to one single variable of childhood adversity and how it relates to functional connectivity changes that can be visualized, and then how it can be used as a factor to predict psychiatric illness. For this review, we included papers from January 1, 2000 – February 28, 2020. All records produced from the search were in English, so we did not have to translate any paper. We focused only on studies that examined connections between two separate regions in the brain and their influence on one another, and on studies that directly visualized the brain using imaging techniques. If a study examined the activity of a single region it was excluded, because functional connectivity by definition must involve the interaction between two regions of the brain. To focus on solely functional connectivity changes in CA, studies related to only structural, morphological, or volumetric alterations were also excluded.
Any studies related to neurochemical alterations that used measurement techniques other than imaging, such as blood tests, were excluded. Any studies related to underlying genetic causes of pathway changes were also excluded. Using these criteria, a total of seventeen studies were found that relate childhood adversity and visualization of functional connectivity changes in neurological circuits (Figure 1).
Figure 1.PRISMA Flow Diagram of Literature Search

Over time attempts to define childhood adversity have been made in order to maintain consistent terminology. However, due to the fact that children have vastly different experiences, it is difficult to have a common dialect with consistent definitions. With the exception of ACE, most terminology is used arbitrarily, although occasionally it reflects which questionnaire was used in a study. Common terms used to describe adversity in childhood include adverse childhood experiences, childhood adversity, early life maltreatment, early life stress, negative childhood experiences, negative stressful life events, parental verbal abuse, witnessing domestic violence, low socioeconomic status, and being bullied
Because there is no uniform definition of adversity, many different study methods are used to categorize and quantify these subjective experiences. Questionnaires are used to create a numerical value from subjective experiences in order to adequately compare subjects. The questionnaires used in the studies reviewed here are the childhood adversity interview,16 childhood trauma questionnaire,17 early life stress questionnaire,18 stressful life events schedule,19 risky family questionnaire,20 verbal abuse questionnaire,21 child and adolescent psychiatric assessment,22 and the Yale-Vermont adversity in childhood scale.23 It is important to recognize that there are spectrums of severity with each questionnaire.
Neural connections in the brain can be examined using a variety of methods, commonly by either diffusion tensor imaging (DTI)24 or magnetic resonance imaging (MRI) and its subtypes.25 DTI looks at the integrity of axons and is used as an approximation of the integrity of myelin.26 DTI uses fractional anisotropy (FA) to assess fasciculi and bundles of axons traveling together. Thus, DTI and FA look at the commissural, projection, and association fibers.27 Specifically, FA assesses myelination by measuring the direction of water movement along axons, and DTI provides a means of visualizing that movement.24 Without detailing the specifics, it should suffice to know that a low FA value signifies a less dense bundle of axons, and an increased FA value signifies a more dense bundle of axons.24 Simply put, white matter integrity is either increased or decreased, and thus is an indicator of connectivity strength.
Nine studies in this review used DTI and FA to look at fasciculi and bundle connections. Three of these studies looked at individuals who had CA and no psychiatric disorders,28–30 and compared them to a control group consisting of adults who had no history of CA and no mental health disorder. Six of these studies looked at patients who had a history of CA and have a psychiatric disorder such as: bipolar disorder,31 major depressive disorder,32 major depressive disorder with bullying,33 schizophrenia,34 substance abuse,30 or trait anger.35 These six studies used control groups of adults with a history of CA but have no psychiatric disorder.
Unlike DTI which examines specific pathways, functional MRI (fMRI) looks at the activation of specific individual regions in the brain. It can additionally be used to visualize connections between regions in the brain,25 which may or may not have an associated specific fasciculi or bundle. The neural activity is measured using a blood oxygen level dependent (BOLD) signal, which can be used to assess functional connectivity. Assessment of inter-regional connections is accomplished by looking at the activity of one region (the seed) and how another region (the target) reacts.36 Functional connectivity is described as either positive or negative; positive connectivity means the seed region activates the target region more, and negative connectivity means the seed region does not activate the target as much. We will be consistent with terms for functional connectivity, where stronger connectivity refers to either more positive or less negative connections; and weaker connectivity refers to either more negative or less positive connections.
Eight studies used MRI and BOLD to look at neural circuitry and functional connectivity between regions in the brain. None of the studies looked at changes in healthy adults; all eight studies looked at adults with a psychiatric disorder who had been exposed to adversity in childhood. These studies used control groups composed of adults who had experienced CA but do not have any psychiatric disorder. The disorders studied were borderline personality disorder,37 major depressive disorder,38 offspring of bipolar parents,39 post-traumatic stress disorder,40 trait anger,41 internalizing symptomatology,42 and externalizing symptomatology.43 Psychiatric disorders can be broadly classified into internalizing and externalizing. Although these terms are not regularly used in practice, they are applied in some studies. Internalizing symptoms include anxiety, depression, somatic symptoms, positive affect, and interpersonal relations. Externalizing symptoms are related to poor impulse control and include drinking alcohol, attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. For each study, Table 1 summarizes the type of adversity, which questionnaire was used, testing conditions, associated mental disorders, and imaging technique used.
Table 1.Assessment of the Methods Used by Each Study
| Questionnaire | Adversity Type | Mental State | Task | Imaging | Reference |
|---|---|---|---|---|---|
| VAS | PVA | No Disorder | Resting state | DTI | Choi et al. 2009 |
| CTQ | WDV | No Disorder | Resting state | DTI | Choi et al. 2012 |
| CAI | ACE | No Disorder, Depression, Substance Abuse | Resting state | DTI | Huang et al. 2012 |
| RFQ | ACE | Bipolar | Resting state | DTI | Benedetti et al. 2014 |
| RFQ | ACE | Depression | Resting state | DTI | Poletti et al. 2018 |
| ELSQ | ELS | Depression + Bullying | Resting state | DTI | Graziano et al. 2019 |
| RFQ | ACE | Schizophrenia | Resting state | DTI | Poletti et al. 2015 |
| CTQ | CA | Trait Anger | Resting state | DTI | Kim et al. 2019 |
| RFQ | ACE | Borderline PD | Negative emotion | fMRI | Vai et al. 2017 |
| CAPA | Poverty | Depression | Resting state | fMRI | Barch et al. 2016 |
| SLES | nSLE | High risk offspring bipolar | Negative emotion | fMRI | Hanford et al. 2019 |
| Direct | Assault | PTSD | Resting state | fMRI | Zielinski et al. 2018 |
| CTQ | CA | Trait Anger | Negative emotion | fMRI | Kim et al. 2018 |
| CTQ | Maltreatment | Internalizing | Positive reward | fMRI | Hanson et al. 2018 |
| CAPA | Longitudinal CA | Externalizing | Resting state | fMRI | Barch et al. 2018 |
| CTQ | Abuse | Externalizing | Negative emotion | fMRI | Peverill et al. 2019 |
| Y-VACS | Maltreatment | Positive support | Negative emotion | fMRI | Wymbs et al. 2020 |
Table 2a and Table 2b are separated by DTI and fMRI studies, and each represents the functional connectivity in each single pathway and the associated disorders. If imaging is obtained, Table 2 is clinically useful because visualizing an affected pathway may help narrow down possibilities of what psychiatric disorders could develop. Another way the data could be organized is by the functional connectivity changes of each single disorder and all the pathways affected. This is useful for research purposes in identifying the pathways affected in a known disorder. However, this is not as clinically relevant because, at the current stage of treatment, the disorder(s) is already diagnosed in a patient and therefore the affected pathways cannot be reversed.
Table 2a.Diffusion Tensor Imaging. Change in Connectivity Strength Arranged by Affected Pathway and Associated Psychiatric Disorders
| Pathway Affected | Connectivity Strength | Mental State | Reference |
|---|---|---|---|
| Diffusion Tensor Imaging and Fractional Anisotropy (FA) | |||
| Arcuate fasciculus (in superior temporal gyrus) | Weaker (Decreased FA) | No Disorder | Choi et al. 2009 |
| Cingulum bundle to cingulate gyrus to hippocampus | Weaker (Decreased FA) | No Disorder, Bipolar, Depression, Schizophrenia, Substance Abuse |
Choi et al. 2009, Benedetti et al. 2014, Poletti et al. 2018, Poletti et al. 2015, Huang et al. 2012 |
| Corona radiata | Weaker (Decreased FA) | Bipolar, Schizophrenia, Trait Anger |
Benedetti et al. 2014, Poletti et al. 2015, Kim et al. 2019 |
| Corona radiata (posterior left) | Stronger (Increased FA) | Depression + Bullying | Graziano et al. 2019 |
| Corpus callosum (genu, body, and splenium) | Weaker (Decreased FA) | No Disorder, Bipolar, Depression, Schizophrenia, Trait Anger |
Huang et al. 2012, Benedetti et al. 2014, Poletti et al. 2018, Poletti et al. 2015, Kim et al. 2019 |
| External capsule | Weaker (Decreased FA) | Trait Anger | Kim et al. 2019 |
| Forceps major | Weaker (Decreased FA) | Depression | Poletti et al. 2018 |
| Forceps minor | Weaker (Decreased FA) | Bipolar, Depression |
Benedetti et al. 2014, Poletti et al. 2018 |
| Fornix body | Weaker (Decreased FA) | No Disorder | Choi et al. 2009 |
| Inferior fronto-occipital fasciculus | Weaker (Decreased FA) | No Disorder, Bipolar, Depression |
Huang et al. 2012, Benedetti et al. 2014, Poletti et al. 2018 |
| Inferior longitudinal fasciculus | Weaker (Decreased FA) | Depression, Schizophrenia, No Disorder |
Poletti et al. 2018, Poletti et al. 2015, Choi et al. 2012 |
| Internal capsule | Weaker (Decreased FA) | Depression | Poletti et al. 2018 |
| Sagittal striatum | Weaker (Decreased FA) | Trait Anger | Kim et al. 2019 |
| Superior fronto-occipital fasciculus | Weaker (Decreased FA) | Trait Anger | Kim et al. 2019 |
| Superior longitudinal fasciculus | Weaker (Decreased FA) | No Disorder, No Disorder, Bipolar, Schizophrenia, Trait Anger |
Huang et al. 2012, Benedetti et al. 2014, Poletti et al. 2018, Poletti et al. 2015, Kim et al. 2019 |
| Thalamic radiation | Weaker (Decreased FA) | Bipolar, Depression, Schizophrenia, Trait Anger |
Benedetti et al. 2014, Poletti et al. 2018, Poletti et al. 2015, Kim et al. 2019 |
| Uncinate fasciculus (amygdala to dlPFC) | Weaker (Decreased FA) | Bipolar, Trait Anger |
Benedetti et al. 2014, Kim et al. 2019 |
| Medial lemniscus | Stronger (Increased FA) | Depression + Bullying | Graziano et al. 2019 |
Functional Magnetic Resonance Imaging. Change in Connectivity Strength Arranged by Affected Pathway and Associated Psychiatric Disorders
| Pathway Affected | Connectivity Strength | Mental State | Reference |
|---|---|---|---|
| Amygdala activity High and no increase dlPFC activity | Weaker (Negative) | Trait Anger | Kim et al. 2018 |
| Amygdala to cerebellum vermis to dorsolateral prefrontal cortex to dorsomedial prefrontal cortex to lateral superior occipital cortex to lingual gyrus to precuneus to subgyral region to superior parietal lobule |
Stronger (Reduced Negative) or (More Positive) | Depression, Borderline PD, High risk offspring bipolar |
Barch et al. 2016, Vai et al. 2017, Hanford et al. 2019 |
| Amygdala to ventral anterior cingulate gyrus to ventral anterior superior frontal gyrus |
Weaker (Reduced Positive) | PTSD | Zielinski et al. 2018 |
| Dorsal anterior cingulate cortex to angular gyrus to lingual gyrus to precuneus |
Weaker (Reduced Positive) | PTSD | Zielinski et al. 2018 |
| Hippocampus to superior frontal cortex | Stronger (Reduced Negative) | Depression | Barch et al. 2016 |
| Inferior frontal gyrus to culmen cerebellum to cuneus to inferior parietal lobule to precentral gyrus |
Weaker (More Negative) | Externalizing | Barch et al. 2018 |
| Rostral anterior cingulate cortex to precuneus rACC to ventral anterior superior frontal gyrus |
Weaker (Reduced Positive) | PTSD | Zielinski et al. 2018 |
| Ventral striatum to medial prefrontal cortex | Stronger (More Positive) | Internalizing (MDD) | Hanson et al. 2018 |
| Ventromedial prefrontal cortex (mOFC & sgACC) to amygdala | Weaker (More Negative) | Externalizing | Peverill et al. 2019 |
| Orbital frontal cortex | Stronger (More Positive) | Positive support | Wymbs et al. 2020 |
| Amygdala | Stronger, not as much | Positive support | Wymbs et al. 2020 |
| Anterior cingulate cortex | (Less increase vs control) | ||
| Frontal pole | |||
| Insula | |||
| Nucleus accumbens (part of ventral striatum) |
Weaker connectivity was observed in nearly every pathway in individuals exposed to CA and was independent of whether the subjects were healthy or had a psychiatric diagnosis.28–35 In total from the DTI studies, sixteen tracts were found to have decreased connectivity and two tracts to have stronger connectivity (Table 2a).
The tracts with stronger connectivity were the medial lemniscus and posterior corona radiata in individuals who were depressed and had a history of being bullied.33 However, the finding of increased connectivity in the left posterior corona radiata33 is opposite to findings from three other studies, which all noted decreased FA in the corona radiata.31,34,35 In particular, two papers specifically noted the left hemisphere was decreased,31,34 and one did not specify laterality.35
Of the tracts with decreased connectivity, nine were associated with multiple disorders (Table 2a). Thus, it cannot be stated that they have a direct correlation to developing a particular disorder if their connectivity strength is decreased. The tracts with decreased connectivity associated with multiple disorders are the cingulum bundle,30–32,34 corona radiata,31,34,35 corpus callosum,31,32,34,35 forceps minor,31,32 inferior fronto-occipital fasciculus,30–32 inferior longitudinal fasciculus,29,32,34 superior longitudinal fasciculus,30–32,34,35 thalamic radiations,31,32,34,35 and the uncinate fasciculus.31,35
Seven of the tracts with decreased connectivity were associated with only a single disorder (Table 2a). The arcuate fasciculus was decreased in adults exposed to adversity but without any disorder.28 The body of the fornix was decreased in subjects without any disorder.28 The external capsule, the sagittal striatum, and the superior fronto-occipital fasciculus were decreased in trait anger.35 The forceps major and the internal capsule were decreased in depression.32
Connections from the amygdala to other regions are altered in trait anger,41 borderline PD,37 depression,38 PTSD,40 and children of bipolar parents at high risk for developing bipolar disorder themselves.39 The amygdala is an emotional control center, thus children who experience adversity are conditioned to control their emotions more, and therefore there could be stronger connections from the amygdala. In support of this, several studies did find stronger connections from the amygdala to multiple other regions including the cerebellar vermis, dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, precuneus, subgyral region,37 lingual gyrus,38 lateral superior occipital cortex, and superior parietal lobule.39
Two studies found weaker connections from the amygdala. One pathway was a less positive BOLD from the amygdala to both the ventral anterior cingulate gyrus and ventral anterior superior frontal gyrus.40 The other pathway was a decreased FA in the uncinate fasciculus from the amygdala to the dorsolateral prefrontal cortex (dlPFC).35 Of note, two studies looked at seed and target region activity in the same pathway from the amygdala to the dorsolateral prefrontal cortex.37,41 They found opposite results, but in different disease states, indicating a possibly pertinent future question. In borderline personality disorder, an increased positive association was found from the amygdala to dlPFC,37 meaning that high amygdala activity results in high dlPFC activity. Contrastingly, in trait anger a negative association was found from amygdala to dorsolateral prefrontal cortex,41 meaning that a high amygdala activity results in less response from the dlPFC. Thus, in both cases the amygdala was hyperactive, but there is a stronger dlPFC response in borderline PD,37 and a weaker dlPFC response in trait anger.41
In normal emotion processing, the amygdala activates the prefrontal cortex (PFC), which feeds back to downregulate the amygdala, thus creating a normal negative association from the PFC to amygdala. One study looked at this feedback response while monitoring subject's negative emotional response; specifically, they looked at the pathway from the ventromedial PFC (vmPFC) to the amygdala.44 They found a weaker (i.e. more negative) connection between the ventromedial PFC to amygdala, indicating that the vmPFC was unable to downregulate the amygdala. Furthermore, they found that the connection became even weaker with an increasing severity of ACE. In other words, children exposed to ACE have less downregulation of the activation of the amygdala.
One study looked at connections form the dorsal anterior cingulate cortex to multiple other regions, and all these pathways were weaker if a subject had been exposed to childhood adversity.40 Several pathways from the inferior frontal gyrus to multiple regions were all found to have more negative connectivity in individuals with externalizing symptomatology.43 The rostral anterior cingulate cortex was found to have weaker connectivity to the precuneus and the ventral anterior superior frontal gyrus in PTSD adolescents.40 These studies indicate that the majority of pathways affected are usually weakened in children exposed to adversity.
Of note there are some single pathway changes that have only been found in one disease. The pathway from the hippocampus to the superior frontal cortex was found to have stronger (i.e. less negative) connectivity in people with depression.38 Stronger connectivity was found from the ventral striatum to the medial prefrontal cortex in people with internalizing symptomatology.42 This could be significant because knowing a single pathway change could help predict the singular disorder outcome; however more research needs to be done on this topic.
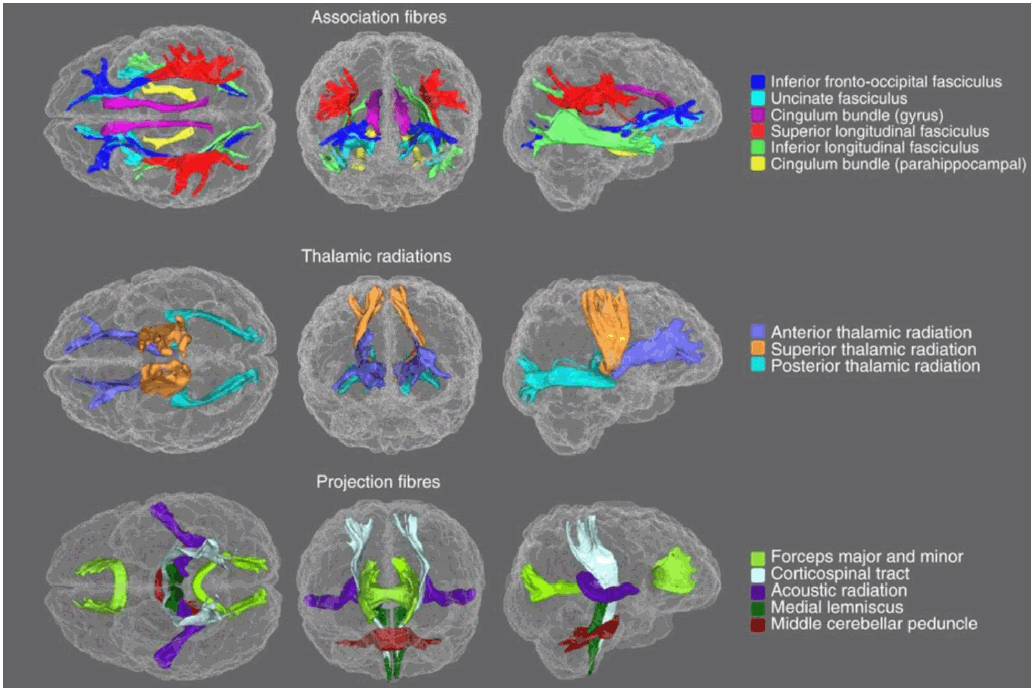
The following is a summary of all the major findings discussed in this review. Stronger connectivity was noted from the hippocampus to the superior frontal cortex in depression,38 from the ventral striatum to the medial prefrontal cortex in depression,42 in the medial lemniscus in people who were bullied with depression,33 and from the amygdala to most target regions in depression,38 borderline PD,37 and offspring of bipolar parents.39 Findings in the corona radiata are unclear because some studies note weaker association,31,34,35 while other studies note stronger connectivity.33 All other pathways had weaker connectivity, including some other pathways from the amygdala. Figure 2 shows white matter tracts,45 most of which are affected by childhood adversity.
Figure 2.White Matter Tracts
Severity of adversity does not have a singular definition, it is a general concept that can relate to how bad a situation is, how many types of adversity one is exposed to, how long the adversity lasts, and how old one is at the time of exposure, among others. Each questionnaire used in the studies provides a spectrum of how severe the adversity was that each child experienced. A common finding across all studies was that the degree of change in the neural circuitry correlated with the severity of adversity. In most tracts, more severe adversity caused more changes in the brain.29,32,34,35,37,44 One effect of this is that the age of onset of psychiatric illness correlates with CA severity. For example, both more severe adversity and longer duration of adversity is associated with an earlier age of onset in bipolar disorder.31 Another finding is that more severe adversity correlates with more severe presentation of a disorder, should it manifest. Increasing severity of adversity increased the severity of borderline personality disorder,37 and increased severity of externalizing symptomatology.43 It should also be noted that increasing severity of adversity positively correlates with both volumetric10 and activity12 changes in the brain.
Considering the results from the DTI studies, it appears that any adversity in childhood can cause a reduction in myelination and reduced connectivity through all major projection, association, and commissural fiber tracts. Furthermore, it can be interpreted that alterations in most of these tracts can cause any disease and are non-specific, meaning that many different tract changes are associated with many different disorders. However, there are a few locations affected that may cause unique disorders. Therefore, it may be more clinically useful to understand connectivity between regions and their excitatory or inhibitory influences. For this reason, the results of the fMRI studies are more clinically useful as they allow the visualization of how one region exerts a response from another region. Clinically, it is also more useful to look at subjects who developed a disorder rather than those who remain healthy. That is to say, understanding the changes in each particular disorder is more important than just understanding changes that do not lead to a disorder.
It is known that people exposed to CA are at higher risk for developing a psychiatric disorder,7 and as mentioned previously, brain changes are positively correlated with increasing severity of adversity. Thus, knowing the severity of adversity that was experienced in childhood may help with preventive medicine. Primary care clinicians should be cognizant of patients who had difficult childhoods and monitor them for development of psychiatric disorder and try to detect early onset. Therefore, in the future it might be an option to recommend that if a patient has a certain number of adverse events (i.e. threshold), they should be monitored more closely for early detection of possible development of psychiatric disorder. Perhaps it should be recommended that new patients take a standardized questionnaire about their childhood experiences.
There is some evidence that the specific type of adversity experienced affects specific regions in the brain involved in those experiences.46 However, there is not a complete data set that indicates exactly what changes are a result of what type of adversity, nor whether there is statistically significant association. Therefore, although research is getting closer, two questions of importance remain unanswered: (1) do certain types of adversity cause certain brain changes? (2) do specific brain changes lead to specific disorders?
If (1) and (2) are both true, then identifying the type of adversity would allow prediction of the type of disorder. This would be especially useful in practice, because it would not require looking at the brain directly to determine what disorder may develop. This provides yet another reason for possibly obtaining a questionnaire about childhood adversity to new patients. If (1) is true but (2) is false, then it can just as appropriately be said that any type of adversity may cause any type of disorder. Apart from already knowing that there is increased risk for a disorder to possibly develop, this would not be especially useful in clinical practice.
If (1) is false but (2) is true, then identifying what change has occurred could allow prediction of what type of disorder might develop in patients with history of severe CA. However, this would require direct visualization of the brain. Therefore, another potential monitor of at-risk patients could be an MRI scan if a threshold number of adverse events has been reached. This would allow for detection of which pathway changes have occurred and therefore narrow the field of potential psychiatric disorders for which they are at risk of developing. Again, this would only be useful if it is determined that certain changes are indeed linked with certain disease states. However, considering the current cost of MRI and cost of care, a thorough cost analysis would need to be performed; this is outside the scope of this paper. A brief look at the cost of care for individuals with history of childhood adversity may be useful. For victims surviving child maltreatment, the associated lifetime medical costs are estimated to be approximately $43,000.47 For child maltreatment resulting in death, the associated lifetime medical costs are approximately $14,000. In 2008, the estimated total lifetime economic burden of childhood maltreatment in the United States was $124 billion and possibly up to $585 billion.47
Possibly the most pertinent question for the future is to determine whether the changes in these children can be prevented entirely, halted, or reversed. More specifically, if children are raised in less adverse environments, or receive treatment (i.e., counseling), can their neural development be unhindered? We should be asking if and how clinicians can assist these children in order to prevent these brain changes. As expected, research is moving in this direction. The study by Wymbs et al. 2020 looked at the effect of how positive support in children exposed to adversity leads to neural changes.48 They found that children who had more positive social support had less of an increase in activity within regions known to be hyperactive in children exposed to adversity.48 This shows that positive support results in less change than expected in children with the same amount of adversity but no positive support. This study is the first to attempt to answer the question of how positive social support affects the neural circuitry of children who had been exposed to adversity. While this study is not the focus of this review, it offers an understanding of the direction in which research will progress and how this knowledge may be applied clinically. This study did not look at connectivity between regions, but rather looked at activity in singular regions.
Other directions for future research include addressing questions such as if clinicians are aware of children experiencing adversity, can that affect the outcome of these children? If primary care physicians were aware of which children experience adversity, would it change how they treat these children? A final question to pursue is to determine which, if any, pathways have not yet been imaged, and obtain information regarding them.
Alternative reasons for these neural changes as a result of CA must be considered. It is possible that these changes are beneficial, not detrimental as many people suggest. When we assume that life is not perfect and all children experience some sort of adversity, then it follows that it should be developmentally important to learn to cope with problems in life, both consciously and unconsciously. Therefore, these neural changes could be adaptive mechanisms to help the individual. This hypothesis is difficult to answer because we have no way to study a control group who have a life without conflict. However, it remains possible that these changes help individuals cope with the world around them and leave them better equipped to handle adversity throughout life.
However, if these changes are beneficial, they do not explain why individuals exposed to CA are more likely to develop psychiatric disorder. Or perhaps because there is already an increased risk of developing a psychiatric disorder, it does not offset the possibility that these neural alterations leave these children better able to deal with their world. In other words, if individuals exposed to childhood adversity are able to maintain normal function, these changes might be beneficial.
One limitation of the studies is related to the composition of study groups. Appropriately each of the studies discussed used childhood adversity (CA) as their group of interest. Control groups had different compositions; sometimes the control was no adversity, other times it was no psychiatric disorder present. In other words, some studies looked at CA vs. no CA, while other studies looked at CA with disorder vs. CA without disorder. The former helps identify changes in everyone exposed to CA but limits insight into clinical outcomes. The latter provides useful information about changes specific to resulting clinical disorders, however there is no completely healthy control group which is a limiting factor.
An additional limitation is that current research suggests most connectivity changes are not specific to any psychiatric disease. In other words, no singular changes have yet been confirmed as a direct link to any specific disease. Also related to this is the limitation of the DTI studies. While they are imperative to understanding and advancing the field, they appear to demonstrate mostly decreased connectivity throughout most of the white matter tracts, and thus do not provide specific changes related to specific disease states.
The final limitation is this review itself. This narrative review helps understand the current stage of research and addresses future directions and possible clinical applications; however, it has a limited comprehensive results analysis. A systematic review would yield more comprehensive data with comparison of results and would also help to identify any bias or random errors.
The more adversity a child experiences, the more likely they are to have negative long-term consequences, such as earlier onset and more severe symptoms of a psychiatric disorder. The underlying mechanism causing these disorders from CA is relatively unknown. Recent research has attempted to answer these questions by mapping functional connectivity throughout the brain. There are many different types of changes in the brain, including alterations in structure, regional activity, circuitry, and functional connectivity. These were thought to be permanent, but the most recent research has begun to demonstrate that they can be attenuated. 48
Clinically, it is important to understand these effects and changes in order to potentially and proactively help these children. Clinicians should be aware that an increasing severity of childhood adversity increases the risk for developing psychiatric disorder. We may be able to identify the development of psychiatric illness earlier and treat it sooner as we continue to increase our understanding of neurological changes. If this can be accomplished, we may also be able to decrease hospitalizations due to mental illness and therefore decrease the overall cost of care while treating more effectively. Learning more about the details of neurological change will help clinicians take better care of their patients. Because childhood adversity increases the risk for both medical disease and psychiatric illness,7,9 primary care practitioners should be on the lookout for development of these disorders. If clinicians are aware that a patient has had severe childhood adversity, they can also be aware of the risk for psychiatric illness and possibly identify the onset earlier. But more importantly, simply having a basic understanding of a patient's childhood experiences could allow for more comprehensive and compassionate care. As we expand this field of knowledge, perhaps new recommendations for primary care practitioners will be implemented. One idea could be to implement a childhood adversity questionnaire for patients in the primary care setting.
This author would like to thank his mentor Dr. James Bruzik.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization, Investigation, Methodology, Writing – Original Draft Preparation, & Writing – Review & Editing: AS.
1. Ujhelyi Nagy A, Kuritár Szabó I, Hann E, Kósa K. Measuring the Prevalence of Adverse Childhood Experiences by Survey Research Methods. International Journal of Environmental Research and Public Health. 2019 Jan;16(6):1048.
2. Soares ALG, Howe LD, Matijasevich A, Wehrmeister FC, Menezes AMB, Gonçalves H. Adverse childhood experiences: Prevalence and related factors in adolescents of a Brazilian birth cohort. Child Abuse Negl. 2016 Jan;51:21–30.
3. Maunder RG, Peladeau N, Savage D, Lancee WJ. The prevalence of childhood adversity among healthcare workers and its relationship to adult life events, distress and impairment. Child Abuse Negl. 2010 Feb;34(2):114–23.
4. Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of Adverse Childhood Experiences From the 2011-2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatr. 2018 Nov;172(11):1038–44.
5. Bürgin D, Boonmann C, Schmid M, Tripp P, O'Donovan A. Fact or artefact? Childhood adversity and adulthood trauma in the U.S. population-based Health and Retirement Study. Eur J Psychotraumatol. 2020 Feb 14;11(1).
6. Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime Prevalence of Mental Disorders in US Adolescents: Results from the National Comorbidity Study-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980–9.
7. Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The Long-Term Health Consequences of Child Physical Abuse, Emotional Abuse, and Neglect: A Systematic Review and Meta-Analysis. PLoS Med. 2012 Nov 27;9(11).
8. Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007 Jan;64(1):49–56.
9. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998 May;14(4):245–58.
10. Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52.
11. Calem M, Bromis K, McGuire P, Morgan C, Kempton MJ. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017;14:471–9.
12. Hein TC, Monk CS. Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment - a quantitative meta-analysis. J Child Psychol Psychiatry. 2017;58(3):222–30.
13. Huffhines L, Noser A, Patton SR. The Link Between Adverse Childhood Experiences and Diabetes. Curr Diab Rep. 2016 Jun;16(6):54.
14. Anderson EL, Fraser A, Caleyachetty R, Hardy R, Lawlor DA, Howe LD. Associations of adversity in childhood and risk factors for cardiovascular disease in mid-adulthood. Child Abuse Negl. 2018 Feb;76:138–48.
15. Traub F, Boynton-Jarrett R. Modifiable Resilience Factors to Childhood Adversity for Clinical Pediatric Practice. Pediatrics. 2017 May 1;139(5).
16. Dienes KA, Hammen C, Henry RM, Cohen AN, Daley SE. The stress sensitization hypothesis: understanding the course of bipolar disorder. J Affect Disord. 2006 Oct;95(1–3):43–9.
17. Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an Adolescent Psychiatric Population. Journal of the American Academy of Child & Adolescent Psychiatry. 1997 Mar 1;36(3):340–8.
18. Cohen RA, Hitsman BL, Paul RH, McCaffery J, Stroud L, Sweet L, et al. Early Life Stress and Adult Emotional Experience: An International Perspective. Int J Psychiatry Med. 2006 Mar 1;36(1):35–52.
19. Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J, et al. The Stressful Life Events Schedule for children and adolescents: development and validation. Psychiatry Research. 2003 Aug 1;119(3):225–41.
20. Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural Responses to Emotional Stimuli Are Associated with Childhood Family Stress. Biological Psychiatry. 2006 Aug 1;60(3):296–301.
21. Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, Stones, and Hurtful Words: Relative Effects of Various Forms of Childhood Maltreatment. Am J Psychiatry. 2006;8.
22. Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA). Psychological Medicine. 1995 Jul;25(4):739–53.
23. Holbrook, H., O'Loughlin, K., Althoff, R., Douglas-Palumberi, H., Kaufman, J., & Hudziak, J. The Yale-Vermont adversity in childhood scale: A quantitative approach to adversity assessment. Paper Presented at the American Academy of Child and Adolescent Psychiatry's 61st Annual Meeting. 2015.
24. Feldman HM, Yeatman JD, Lee ES, Barde LHF, Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatr. 2010 May;31(4):346–56.
25. Chen K, Azeez A, Chen DY, Biswal BB. Resting-State Functional Connectivity: Signal Origins and Analytic Methods. Neuroimaging Clin N Am. 2020 Feb;30(1):15–23.
26. Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. NeuroImage. 2002 Nov 1;17(3):1429–36.
27. Duarte JA, de Araújo E Silva JQ, Goldani AA, Massuda R, Gama CS. Neurobiological underpinnings of bipolar disorder focusing on findings of diffusion tensor imaging: a systematic review. Braz J Psychiatry. 2016 Mar 22;38(2):167–75.
28. Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009 Feb 1;65(3):227–34.
29. Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. Neuroimage. 2012 Jan 16;59(2):1071–9.
30. Huang H, Gundapuneedi T, Rao U. White Matter Disruptions in Adolescents Exposed to Childhood Maltreatment and Vulnerability to Psychopathology. Neuropsychopharmacology. 2012 Nov;37(12):2693–701.
31. Benedetti F, Bollettini I, Radaelli D, Poletti S, Locatelli C, Falini A, et al. Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychol Med. 2014 Oct;44(14):3069–82.
32. Poletti S, Aggio V, Brioschi S, Bollettini I, Falini A, Colombo C, et al. Impact of early and recent stress on white matter microstructure in major depressive disorder. J Affect Disord. 2018 01;225:289–97.
33. Graziano RC, Bruce SE, Paul RH, Korgaonkar MS, Williams LM. The effects of bullying in depression on white matter integrity. Behav Brain Res. 2019 02;363:149–54.
34. Poletti S, Mazza E, Bollettini I, Locatelli C, Cavallaro R, Smeraldi E, et al. Adverse childhood experiences influence white matter microstructure in patients with schizophrenia. Psychiatry Res. 2015 Oct 30;234(1):35–43.
35. Kim MJ, Elliott ML, d'Arbeloff TC, Knodt AR, Radtke SR, Brigidi BD, et al. Microstructural integrity of white matter moderates an association between childhood adversity and adult trait anger. Aggress Behav. 2019;45(3):310–8.
36. Smitha KA, Akhil Raja K, Arun KM, Rajesh PG, Thomas B, Kapilamoorthy TR, et al. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017 Aug;30(4):305–17.
37. Vai B, Sforzini L, Visintini R, Riberto M, Bulgarelli C, Ghiglino D, et al. Corticolimbic Connectivity Mediates the Relationship between Adverse Childhood Experiences and Symptom Severity in Borderline Personality Disorder. Neuropsychobiology. 2017;76(2):105–15.
38. Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester C, et al. Hippocampal and Amygdala Connectivity Mediate The Relationship Between Preschool Poverty and School Aged Depression. Am J Psychiatry. 2016 Jun 1;173(6):625–34.
39. Hanford LC, Eckstrand K, Manelis A, Hafeman DM, Merranko J, Ladouceur CD, et al. The impact of familial risk and early life adversity on emotion and reward processing networks in youth at-risk for bipolar disorder. PLoS ONE. 2019;14(12):e0226135.
40. Zielinski MJ, Privratsky AA, Smitherman S, Kilts CD, Herringa RJ, Cisler JM. Does development moderate the effect of early life assaultive violence on resting-state networks? An exploratory study. Psychiatry Res Neuroimaging. 2018 30;281:69–77.
41. Kim MJ, Scult MA, Knodt AR, Radtke SR, d'Arbeloff TC, Brigidi BD, et al. A Link Between Childhood Adversity and Trait Anger Reflects Relative Activity of the Amygdala and Dorsolateral Prefrontal Cortex. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(7):644–9.
42. Hanson JL, Knodt AR, Brigidi BD, Hariri AR. Heightened connectivity between the ventral striatum and medial prefrontal cortex as a biomarker for stress-related psychopathology: understanding interactive effects of early and more recent stress. Psychol Med. 2018;48(11):1835–43.
43. Barch DM, Belden AC, Tillman R, Whalen D, Luby JL. Early Childhood Adverse Experiences, Inferior Frontal Gyrus Connectivity, and the Trajectory of Externalizing Psychopathology. J Am Acad Child Adolesc Psychiatry. 2018;57(3):183–90.
44. Peverill M, Sheridan MA, Busso DS, McLaughlin KA. Atypical Prefrontal-Amygdala Circuitry Following Childhood Exposure to Abuse: Links With Adolescent Psychopathology. Child Maltreat. 2019;24(4):411–23.
45. Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, et al. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nature Communications. 2016 Dec 15;7(1):13629.
46. Teicher MH, Samson JA. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016 Mar;57(3):241–66.
47. Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 2012 Feb;36(2):156–65.
48. Wymbs NF, Orr C, Albaugh MD, Althoff RR, O'Loughlin K, Holbrook H, et al. Social supports moderate the effects of child adversity on neural correlates of threat processing. Child Abuse Negl. 2020 Apr;102:104413.
Alexander L. Shand, 1 Honours Bachelor of Science, University of Toronto, Canada. St. Matthew's University School of Medicine, Grand Cayman, Cayman Islands
About the Author: Alec Shand is a fourth-year medical student at St. Matthew's University School of Medicine. He was a recipient of the Dr. John Randall Scholarship and is an Eagle Scout. He enjoys competitive sportfishing and coaching middle school lacrosse and ice hockey.
Correspondence: Alexander Shand, Address: 12124 High Tech Avenue, Suite 290, Orlando, USA. Email: Ashand@stmatthews.edu
Editor: Kelly R. MacArthur & Francisco J. Bonilla-Escobar Student Editors: Madeleine J. Cox, Samreen Fathima, & Joseph Tonge Copyeditor: Ciara Egan Proofreader: Ciara Egan Layout Editor: Francisco J. Bonilla-Escobar
Copyright © 2021 Alexander Shand
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 9, NUMBER 1, April 2021