Fixed Drug Eruption due to Vildagliptin-Metformin Fixed Dose Combination Tablet.
Highlights:Jessica Kaushal1, Abhimanyu Rakesh2
doi: http://dx.doi.org/10.5195/ijms.2020.646
Volume 8, Number 3: 291-294
Received 10 07 2020: Rev-request 06 09 2020: Rev-recd 08 09 2020: Accepted 24 09 2020
ABSTRACT
Background:A fixed drug eruption is a type IV hypersensitivity reaction to a medication that characteristically re-emerges on the same site each time the specific drug is taken. Antimicrobials (including fixed dose combinations) are frequently implicated in fixed drug eruption while gliptins (as separate drugs or as combined preparations) on the other hand are infrequent triggers. Drugs belonging to similar classifications and having similar chemical structures can show cross reactivity, but here we describe a case of cross reactivity between unrelated drug classes, also known as polysensitivity.
The Case:A 58-year-old man presented with painful, burning, and pruritic blisters with ulcerations on the oral mucosa of his lips, hard palate, and tip of the tongue. The patient had been on vildagliptin - metformin fixed dose combination tablets for one year. He was asked to stop the drug and lesions started improving thereafter. A week later he suffered from gastroenteritis for which he took a combined preparation of ofloxacin - ornidazole and lesions re-appeared at the same site as before with severe itching and burning.
Conclusion:This case highlights polysensitivity amongst chemically unrelated drugs, especially available in fixed dose combination. It is an extremely rare occurrence (less than 0.2%). Moreover, there have only been a few cases of such delayed reactions occurring to gliptins, especially vildagliptin. A clinician must keep a high index of suspicion to identify this phenomenon.
Keywords: Drug Eruptions; Delayed Hypersensitivity; Gliptins; Antimicrobial agents; Titanium dioxide (Source: MeSH-NLM).
Fixed drug eruption (FDE) is a cell mediated type IV hypersensitivity reaction frequently seen with antimicrobials, anticonvulsants and non-steroidal anti-inflammatory drugs (NSAIDs).1 FDE is characterized by well-defined macular eruptions associated with blistering, burning or pruritis.1 Cross reactivity is frequently seen between drugs belonging to the same class. The lesions recur every time an offending agent is taken. Resolution of lesions is accompanied by a dusky pigmentation due to post-inflammatory disordered melanin distribution between keratinocytes. This hyperpigmentation is permanent in character.1 The condition becomes lifelong and avoidance of the offending agent is the key to management.1
Type-2 diabetes mellitus is a prevalent chronic disease and the most common cause of significant morbidity around the world. Metformin, a biguanide, is the first line agent to which sulfonylureas (SUs) and dipeptidyl peptidase-4 inhibitors (DPP-4i) can be added as second line agents as the disease progresses.2 In the last decade, prescribing gliptins with metformin as an initial combination therapy is becoming a popular practice in India due to comparatively rapid achievement of target blood glucose levels with a negligible risk of hypoglycemia as the incretin effect of gliptins is glucose dependent.3 The additive effect of these two drugs also enhances insulin sensitivity.3 DPP-4i are more promising than SUs due to a better side effect profile along with better control on weight when added to metformin.4 Vildagliptin is a potent DPP-4i and is frequently prescribed in type-2 diabetics as an add on second line agent when the metformin monotherapy fails.5 Several adverse reactions have been attributed to this drug; however, reports regarding FDEs with gliptins have been very few to date.6
In this case, the patient had already been on vildagliptin-metformin (V-MF) 50mg/1000mg twice daily for almost one year when he started experiencing perioral itching with oral mucosal blistering and ulcerations. After identifying the potential of V-MF as a trigger for these reactions, the patient was asked to stop the drug, after which he achieved remission of his oral lesions. One week later, the patient was prescribed ofloxacin-ornidazole (OFL-ONZ) 200mg/500mg for an episode of gastroenteritis, and the re-emergence of similar oral lesions occurred at the same previously involved site but this time with exaggerated symptoms of itching, burning and blistering. This case is unique for not only reporting an infrequent adverse event associated with DPP-4 inhibitors (gliptins) but also for focusing on polysensitivity of FDEs in chemically unrelated classes of medications, particularly those prescribed in fixed dose combination (FDC) tablets. The main aim of this article is to address an adverse event of polysensitivity which may be attributed to the common excipient used in the coating of these FDC tablets.
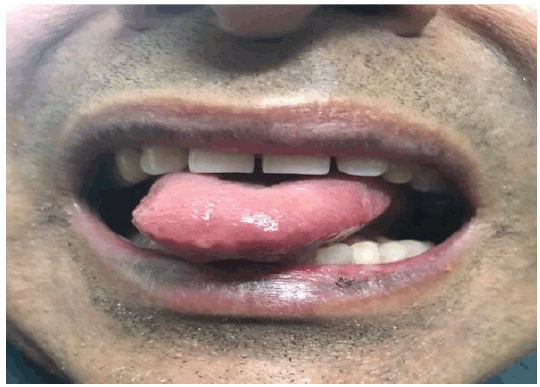
A 58-year-old man presented in the clinic with chief complaints of itching, burning, and appearance of blisters and ulcerations with erythema on the oral mucosa of his lips, hard palate, and tip of the tongue for the past 2 days. The lesions appeared within 2-3 hours after consuming breakfast in the morning 2 days ago. The patient assumed the episode to be an allergic response to fruits he had consumed in his breakfast and decided to omit fruits in his following meals. He took fexofenadine hydrochloride, an over-the-counter antihistamine, for the lesions but there was no relief. The patient presented 2 days later due to persistence of these lesions despite the steps taken (Figure 1).
Figure 1Fixed Drug Eruption due to Vildagliptin-Metformin Fixed Dose Combination Tablet.

He had no previous history of allergic reactions. Medical history was positive for diabetes mellitus type 2. He used to drink alcohol occasionally. Drug history included V-MF FDC oral preparation (50mg/1000mg, twice daily after meals) for the past year. He reported consuming the tablet from a new blister pack immediately after his breakfast a couple of days before presenting to the clinic. He continued to take the drug for the next 2 days as well. He was asked to discontinue the drug and follow up after 2 days. The patient claimed that he experienced relief in the following 48 hours after discontinuing the drug.
An oral drug provocation test (DPT) was performed with V-MF full dose (1 tablet) which led to itching, burning and ulceration around the previous lesions. The positive test therefore supported the diagnosis of FDE due to this FDC drug. He switched to metformin and vildagliptin (as separate tablets) thereafter and did not have any problem. One week later, the patient experienced an episode of gastroenteritis with acute onset diarrhea for which he was administered OFL-ONZ, a FDC oral preparation containing ofloxacin (200mg) and ornidazole (500mg). Soon after this, there was recurrence of itching, erythema and burning around the lips. On discontinuation of OFL-ONZ, lesions started healing. Two days later, a DPT with half the dose of OFL-ONZ, the itching re-emerged around the lips. The patient claimed no such reaction occurring with OFL-ONZ in the past. He was then advised to abstain from these as well as any FDC preparations in future.
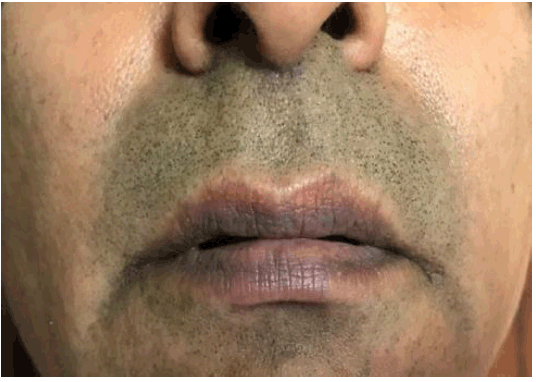
Two months after cessation of V-MF and refraining from FDC medications, the skin eruptions had subsided but there was a residual dark pigmentation on both the upper and lower lips where the original lesions had existed (Figure 2).
Figure 2Residual Hyperpigmentation 10-Weeks After Cessation of the Offending Drugs
Vildagliptin is a potent DPP-4i that prevents breakdown of endogenous incretins by DPP-4 enzyme thereby enhancing insulin secretion.
Extensive monotherapy trials have shown improved glycemic regulation and substantial glycated hemoglobin (HbA1c) reductions in type-2 diabetics along with a favorable adverse effect profile.7 These features make vildagliptin a propitious agent for combination therapies (especially with metformin) which has been evidenced through clinical trials.7 Metformin, a biguanide, has a different target of action. Both drugs are highly effective first line agents. Over time, regimens in diabetics require adjustments and new drug combinations to target different metabolic problems emerging within the body. FDCs help to simplify the already complex medication regimen in diabetics and helps improve patient compliance.
The rationale behind combining fixed doses of two different drugs with different mechanisms of action is the benefit of an additive effect and a smaller number of individual doses required to achieve that effect.8 As a result, vildagliptin (V) and metformin (MF) are frequently prescribed in FDCs (e.g. V-MF, an FDC drug combing these two salts in the ratio of 50mg:1000mg, respectively). V-MF is often prescribed twice daily along with non-pharmacological interventions such as lifestyle modifications (exercise, diet control) to keep blood sugar adequately controlled. Ofloxacin, a potent fluroquinolone is effective against gram-negative and gram-positive bacteria covering both Staphylococcus Aureus and Pseudomonas Aeruginosa while ornidazole, a nitro imidazole derivative, is both an antibacterial and antiprotozoal. With a similar rationale, antimicrobials are also often prescribed in FDCs to hit several targets with a single dose and provide an additive effect. In addition, FDCs help to overcome antibiotic resistance and increase the antimicrobial spectrum.9
Drug allergies can occur at any time. Here the patient developed a delayed-type hypersensitivity reaction after 1 year of taking V-MF FDC. An FDE is a type-IV hypersensitivity reaction to a medication, that characteristically re-emerges on the same site each time the drug is taken.10 Cross-reactivity between structurally or chemically similar drugs may occur. The lesions often involve the skin (trunk, limbs, etc.) and mucosal surfaces (lips, genitalia, perianal region) and may be localized or generalized. Healing begins once the offending agent is stopped, which is often followed by persistent dusky brown or purple post-inflammatory hyperpigmentation at the site of the reaction. This is called post-inflammatory hyperpigmentation that occurs once the inflammation subsides and is due to increased melanin production along with disordered distribution of melanin to the surrounding keratinocytes via melanosomes.11 There have been very few reports regarding FDEs secondary to gliptins and metformin.12 There has been no reported case of V-MF FDC drug-induced FDE to our knowledge. On the other hand, OFL-ONZ FDC induced FDE have been quite frequently reported in the literature; however, polysensitivity (i.e. two structurally and chemically unrelated drugs inducing the same allergic reaction at the same site) has almost never been reported. Shiohara et al. once commented on an unusual case where polysensitivity was seen amongst three anticonvulsants.13 He proposed that such phenomenon exists frequently in reality but is under-reported due to lack of awareness and suspicion, and it is possible that polysensitivity is due to a non-antigen specific phenomenon.13
In this case, the patient exhibited a polysensitivity phenomenon where two different FDC drugs induced hypersensitivity reactions at the same site. This was supported by the DPTs as well. DPT is the supervised administration of a suspected allergen to accurately diagnose hypersensitivity reactions. The patient did not experience any such reaction when individual drugs as separate salt-formulations were given. On reviewing the composition of all these drugs i.e., vildagliptin, metformin, their FDC preparation, ofloxacin, ornidazole and their FDC preparation, our attention was drawn towards the inactive inorganic ingredients. FDC drugs are usually coated with titanium dioxide nanoparticles (TiO2NPs) while the uncombined drugs come in an uncoated form. This has also been confirmed by spectroscopic studies.14 Titanium dioxide (TiO2) is a Food and Drug Administration approved food and pharmaceutical additive, mainly to confer white opaque coating to confectionaries, dairy products, and medicine tablets. TiO2NPs toxicity has not yet been established due to lack of sufficient research and reporting. TiO2, as a macroparticle or microparticle, is generally not harmful; however, toxicity due to TiO2 nanoparticles is a novel concern in biomedical research. In a recent study it was said that nanoparticles (defined as particles with diameter <100 nm) become a health concern, especially if their size is 30 nm or less as they are not phagocytosed effectively by phagocytes, and can potentially trigger a pro-inflammatory cascade causing free radical injury.15 Also, a relatively small size of nanoparticles means higher surface area to volume ratio which potentially promotes nanoparticle deposition in tissues.15 As an opacifying pigment, TiO2 particle size spans over a range of 25 nm – 300 nm.16 Since TiO2 powders contain a variety of sizes, a fraction of nanoparticles does exist (which includes particles <30 nm in size) and it is roughly up to 36%.16 A review study by Skocaj et al. mentions that although TiO2NPs are considered safe, data on their toxic effects upon oral exposure has been very scarce. They propose that TiO2, when exposed to the acidic pH of the stomach, gets solubilized and disseminated via the blood and lymph into the different tissues especially the liver, spleen, kidneys and lungs.17 Although the amount of TiO2 consumed via foods and medicines is far below the levels used for experimental studies that were reviewed to study the impact of TiO2NPs on the intestinal microenvironment and systemic immunoregulation,18 there still stands a possibility of the cumulative effect of TiO2 being consumed in small doses and therefore being accumulated constantly over long periods of time and triggering adverse reactions.18 However, there is a paucity of research in this domain.
FDEs are infrequently associated with antidiabetic agents as very few cases have been reported and none have been reported with FDC preparation of vildagliptin and metformin to our knowledge. Healthcare providers should be aware of such rare adverse events with these drugs. Polysensitivity, especially amongst FDC preparations is possible. This case also discusses the possibility of TiO2 being a trigger antigen for type-IV hypersensitivity drug reactions as this patient experienced FDE with drugs containing TiO2 as an excipient (inactive ingredient), but did not experience a reaction when he consumed the same drugs in uncombined preparations that lacked TiO2.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization, Data Curation, Investigation, Methodology, Resources, and Visualization: JK & AR. Project Administration: JK. Validation: AR. Writing – Original Draft Preparation: JK. Writing – Review & Editing: JK & AR.
1. Jhaj R, Chaudhary D, Asati D, Sadasivam B. Fixed-drug Eruptions: What can we Learn from a Case Series? Indian J Dermatol. Jul-Aug 2018;63(4):332-337.
2. Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012 Apr;38(2):89-101.
3. Ahrén B. Novel combination treatment of type 2 diabetes DPP-4 inhibition + metformin. Vasc Health Risk Manag. 2008 Apr;4(2):383-94.
4. Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012 Mar 12;344: e1369.
5. Lukashevich V, Del Prato S, Araga M, Kothny W. Efficacy and safety of vildagliptin in patients with type 2 diabetes mellitus inadequately controlled with dual combination of metformin and sulphonylurea. Diabetes Obes Metab. 2014 May;16(5):403-9.
6. Stein SA, Lamos EM, Davis SN. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin Drug Saf. 2013 Mar;12(2):153-75.
7. Mathieu C, Degrande E. Vildagliptin: a new oral treatment for type 2 diabetes mellitus. Vasc Health Risk Manag. 2008;4(6):1349-60.
8. Moon C, Oh E. Rationale and strategies for formulation development of oral fixed dose combination drug products. Int J Pharm Investig. 2016;46:615-31.
9. Tyers M, Wright GD. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol. 2019 Mar;17(3):141-155.
10. Korkij W, Soltani K. Fixed drug eruption. A brief review. Arch Dermatol. 1984 Apr;120(4):520-4.
11. Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010 Jul;3(7):20-31.
12. Nakatani K, Kurose T, Hyo T, Watanabe K, Yabe D, Kawamoto T, et al. Drug-induced generalized skin eruption in a diabetes mellitus patient receiving a dipeptidyl peptidase-4 inhibitor plus metformin. Diabetes Ther. 2012 Dec;3(1):14.
13. Chan HL, Tan KC. Fixed Drug Eruption to Three Anticonvulsant Drugs: An Unusual Case of Polysensitivity. J Am Acad Dermatol. 1997 Feb;36(2 Pt 1):259.
14. Myakalwar AK, Sreedhar S, Barman I, Dingari NC, Venugopal Rao S, Prem Kiran P, et al. Laser-induced breakdown spectroscopy-based investigation and classification of pharmaceutical tablets using multivariate chemometric analysis. Talanta. 2011 Dec 15;87:53-9.
15. Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR, et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009 Oct;4(10):634-41.
16. Peters RJB, van Bemmel G, Herrera-Rivera Z, Helsper HPFG, Marvin HJP, Weigel S, et al. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J Agric Food Chem. 2014 Jul 9;62(27):6285-93.
17. Skocaj M, Filipic M, Petkovic J, Novak S. Titanium dioxide in our everyday life; is it safe? Radiol Oncol. 2011 Dec;45(4):227-47.
18. Bettini S, Boutet-Robinet E, Cartier C, Coméra C, Gaultier E, Dupuy J, et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci Rep. 2017 Jan 20;7:40373.
Jessica Kaushal, 1 MBBS, Intern, Government Medical College, Amritsar; Baba Farid University of Health Sciences, Faridkot, Punjab, India
Abhimanyu Rakesh, 2 MBBS, Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar; Baba Farid University of Health Sciences, Faridkot, Punjab, India
About the Author: Jessica Kaushal is an intern rotating at Guru Nanak Dev Hospital, Amritsar, Punjab, India. She has presented a paper at a national conference organized by Junior World Congress. She rotated at Cardiology department, Unicardio, Florida International University, Miami this year and has expressed her keen interest in Internal Medicine. She aspires to pursue her residency in the United States. Dr. Abhimanyu Rakesh is currently working as a resident medical officer at Magnus Hospital, Panchkula, Haryana, India. He recently graduated from Sri Guru Ram Das Institute of Medical Sciences and Research, Amritsar. He has rotated in the United States for 3 months to gain hands on clinical experience and has expressed his appreciation for the US clinical set up.
Correspondence: Jessica Kaushal. Address: Majitha Rd, Medical Enclave, Amritsar, Punjab 143001, India. Email: jessicakaushal1995@gmail.com
Editor: Ammar Ismail Student Editors: Nikoleta Tellios, Adam Dinoff & Mohamed Fahmy Doheim Copyeditor: Sohaib Haseeb Proofreader: Nicole Katherine Conners Layout Editor: Annora A. Kumar
Cite as: Kaushal J, Rakesh A. Fixed Drug Eruption: A Rare Case of Polysensitivity between Two Unrelated Fixed Dose Combination Preparations - A Case Report. Int J Med Students. 2020 Sep-Dec;8(3):291-4.
Copyright © 2020 Jessica Kaushal, Abhimanyu Rakesh
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 8, NUMBER 3, December 2020