Reviews
Novel Combination Strategies to Enhance Immune Checkpoint Inhibition in Cancer Immunotherapy:
A Narrative Review
Jonathan A. Hermel1, Cassi M. Bruni2, Darren S. Sigal3
doi: http://dx.doi.org/10.5195/ijms.2020.753
Volume 8, Number 3: 273-280
Received 14 09 2020:
Rev-request 16 10 2020:
Rev-recd 008 11 2020:
Accepted 24 11 2020
ABSTRACT
Programmed cell death protein-1 (PD-1) is an immune checkpoint receptor that induces
and maintains tolerance of T cells, invariant natural killer T (iNKT) cells, and natural
killer (NK) cells, among other lymphocytes. Immune checkpoint inhibition by PD-1 blockade
restores the lymphocytic immunostimulatory phenotype and has been successful in the
treatment of various malignancies. However, while immune checkpoint blockade has been
shown to provide robust antitumor treatment outcomes, its overall response rate remains
low in a significant portion of cancer patients. An essential unmet need in cancer
therapy is the development of novel pharmacologic strategies designed to lower rates
of resistance associated with immune checkpoint blockade. Therefore, efforts that
seek to enhance the efficacy of PD-1 inhibition possess profound immunotherapeutic
potential. Here, three promising combination strategies that harness the antitumor
effects of immune checkpoint inhibitors (ICIs) together with non-ICI antitumor therapeutic
agents are reviewed. These agents include (1) ABX196, a potent inducer of iNKT cells,
(2) chimeric antigen receptor (CAR)-T cell therapy, and (3) NK cell therapy. A comprehensive
literature search was conducted using the PubMed and ClinicalTrials.gov databases for scientific articles and active trials, respectively, pertaining to
immune checkpoint inhibition, iNKT cells, CAR-T cells, and NK cell immunotherapy.
Preliminary clinical and preclinical data suggest that these combination treatment
regimens greatly suppress tumor growth and may serve as innovative methods to enhance
and optimize anticancer immunotherapy.
Keywords:
Immunotherapy;
Immune checkpoint molecules;
Invariant Natural Killer T Cell;
Chimeric Antigen Receptor;
Natural Killer T-Cells (Source: MeSH-NLM).
Introduction
Immunotherapy represents the newest pillar in anticancer therapy. The first fifty
years of anticancer therapy consisted solely of cytotoxic chemotherapy, but this began
to change in the 1990s with the advent of monoclonal antibodies and again in the early
2000s with the development of small molecule tyrosine kinase inhibitors. Each successive
new approach has increased therapeutic efficacy and resulted in improved patient outcomes.
United States Food and Drug Administration (FDA)-approved immunotherapies, in the
form of immune checkpoint inhibitors (ICIs) to the cytotoxic T-lymphocyte-associated
protein-4 (CTLA-4) and programmed cell death protein-1 (PD-1) checkpoint receptors,
may represent the most profound advances in anticancer therapy in modern history.
Cancers notoriously difficult to manage, including non-small cell lung cancer (NSCLC)
and melanoma, have responded well to ICI immunotherapy, whether administered solely
as monotherapy or in combination with chemotherapy in the neoadjuvant, adjuvant, and
advanced late-stage settings.1,2,3 However, despite their proven antitumor treatment outcomes, ICIs have modest overall
response rates, not only between varying cancer types, but also among patients who
share the same malignancy.4 The identification of novel tumor biomarkers is an ongoing area of research aimed
at predicting resistance to ICI immunotherapy and developing targeted approaches that
seek to overcome this resistance.5 It remains that a key unmet need in cancer therapy is improving the consistency and
functional efficacy of ICIs in a majority of cancer patients. A variety of novel immune
therapies and targeting approaches are in clinical development that may mark another
important step forward towards this goal. This review will examine the preclinical
and clinical data of ABX196, a non-checkpoint inducer of invariant natural killer
T (iNKT) cells; chimeric antigen receptor (CAR)-T cell therapy; and natural killer
(NK) cell therapy, each in combination with ICIs for improved anticancer immunotherapy.
Methods
An extensive scientific literature search was performed using the PubMed database
for peer-reviewed articles published in academic journals related to immune checkpoint
inhibition, iNKT cells, CAR-T cells, and NK cells. The literature search was conducted
between the months of May and July 2020. Search parameters included combinations of
the keywords “ABX196,” “α-GalCer,” “invariant natural killer T cell,” “iNKT cell,”
“chimeric antigen receptor T cell,” “CAR-T cell,” “natural killer cell,” or “NK cell,”
with the terms “PD-1,” “immune checkpoint inhibitor,” “immune checkpoint inhibition,”
or “immunotherapy.” Only studies published in English with full available text were
screened for use. Because of the intended comprehensive nature of this review, a small
portion of the manuscript pertaining to the background and history of α-GalCer mechanistic
studies includes original articles published from 1994 to 2018. For the remainder
of the paper, only articles published within the past 10 years were considered for
use. Article abstracts were closely reviewed for applicability to the research question
and those without clear relevance were removed from consideration. For information
regarding active clinical trials, a detailed search of ClinicalTrials.gov was conducted. Initial search parameters included the keyword phrases, “ABX196,”
“CAR-T cell,” “NK cell” or “natural killer cell.” Trials located in all countries
were included for review; however, trials that were not in the recruiting or pre-recruiting
stages were filtered out. Listed trial titles and descriptions were then manually
screened for protocols that included additional ICI immunotherapies. Trials that did
not meet the aforementioned criteria were excluded.
Results and Discussion
ABX196 in Combination with PD-1 Blockade
Background and rationale: iNKT cell anergy is associated with PD-1 upregulation
ABX196 is a synthetic glycolipid analogue of α-galactosylceramide (α-GalCer), a strong
agonist of invariant natural killer T (iNKT) cells. In contrast to conventional T
and NK lymphocytes, iNKT cells induce both innate and adaptive antitumor immune responses.6 iNKT cells express an invariant T cell receptor (TCR) composed of Vα14-Jα18/Vβ8.2
gene chain rearrangements in mice and Vα24-Jα18/Vβ11 gene chain rearrangements in
humans.7 These invariant TCRs recognize endogenous and exogenous lipid moieties bound to and
presented on non-classical, major histocompatibility complex (MHC) class I-like CD1d
molecules.8 CD1d is a membrane-bound cell surface glycoprotein expressed primarily by B lymphocytes,
macrophages, and dendritic cells (DCs).9 When lipid moieties are presented within the hydrophobic binding-groove of CD1d,
they interact with the iNKT TCR; this association stimulates iNKT cells, which in
turn modulate the immune system.10 Specifically, in vivo administration of α-GalCer in mice triggers iNKT cells to i) rapidly proliferate,
ii) release a variety of cytokines, including IFN-γ and IL-4, iii) cross-prime dendritic
cells to release IL-12, and iv) slow tumor growth and prevent metastasis.11,12 Although α-GalCer is a powerful iNKT cell-stimulating antigen, its antitumor efficacy
is restricted by the fact that α-GalCer-stimulated iNKT cells release cytokines with
opposing immune system actions, specifically IFN-γ, which initiates an immunostimulatory
T helper 1 (TH1) response, and IL-4, which initiates an immunoregulatory T helper
2 (TH2) response.13 The favorable effect of iNKT cells in antitumor immunity is due, in large part, to
IFN-γ production.14 ABX196 consists of an acetamide group linked to the galactosyl C6 of α-GalCer, and
this structural modification has been shown to enhance the secretion of TH1 cytokines.15 As compared to α-GalCer, ABX196 produces high levels of systemic IFN-γ but significantly
lower levels of IL-4, confirming a pro-inflammatory TH1 skew in cytokine production.15
While ABX196 induces a more potent immunostimulatory response beneficial for antitumor
immunity, iNKT cell activation quickly leads to a long term unresponsive, anergic
state for two primary reasons: one, the iNKT TCR becomes downregulated;16,17 and two, PD-1 receptors become upregulated at the surface of iNKT cells.18,19 PD-1 is a T cell checkpoint receptor that, when bound to its ligand PD-L1, functions
to induce and maintain T cell tolerance (Figure 1).20 The FDA has approved a number of monoclonal antibodies that target and interfere
with the PD-1/PD-L1 signaling pathway for use in cancer therapy.21 These include anti-PD-1 antibodies nivolumab, pembrolizumab, and cemiplimab, as well
as anti-PD-L1 antibodies atezolizumab, avelumab, and durvalumab.21 The aforementioned ICIs have emerged in recent years as effective therapeutics for
a variety of cancers due to their unique ability to block and reverse T cell anergy.22 These findings have led to the pharmaceutical approach of simultaneously administering
both an iNKT cell agonist and an anti-PD-1 antibody to limit iNKT cell anergy and
thus enhance antitumor immunity.
Figure 1
Mechanism of Checkpoint Inhibition in Promoting Anti-Tumor Immune Stimulation
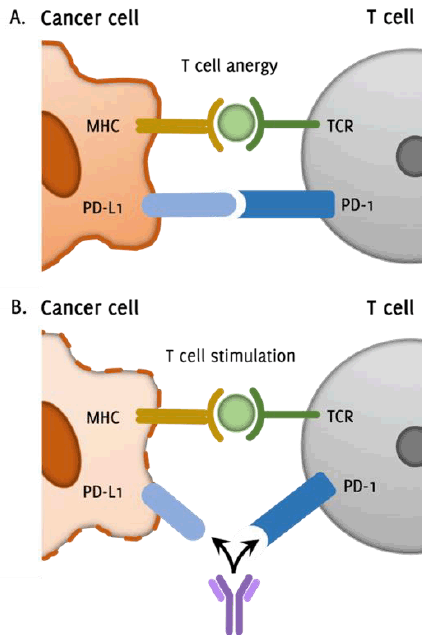
Legend: Simplified illustration demonstrating the mechanism of immune checkpoint inhibition
in cancer immunotherapy. Figure 1.A. shows the T cell programmed cell death protein-1 (PD-1) receptor interacting with
the corresponding tumor programmed cell death protein-1 ligand (PD-L1) receptor, leading
to T cell anergy and inactivity. This occurs despite appropriate antigen presentation
on the major histocompatibility complex (MHC) and proper recognition by the T cell
receptor (TCR). Figure 1.B. demonstrates the targeted interruption of the PD-1/PD-L1 interaction by a monoclonal
antibody immune checkpoint inhibitor (ICI). This inhibition of PD-1/PD-L1 signaling
promotes continued stimulation of the T cell to induce tumor cell death.
Preclinical Data: iNKT Agonist with PD-1 Blockade
Several preclinical studies have explored the immunotherapeutic potential of α-GalCer-mediated
iNKT induction in combination with PD-1 blockade. Parekh et al.19 demonstrated that antibody-mediated inhibition of PD-1/PD-L1 interactions at the
time of α-GalCer treatment prevented the induction of iNKT cell anergy and enhanced
the anti-metastatic activity of α-GalCer in wild-type mouse models. The same study
showed that PD-1 deficient mice were resistant to α-GalCer-induced iNKT cell anergy.
Durgan et al. 23 performed similar experiments with murine melanoma models. In their study, PD-L1
deficient mice were administered DCs loaded with antigen and α-GalCer; these mice
subsequently had a significant reduction in tumor size associated with increased trafficking
of antigen-presenting cells (APCs) and CD8+ cytotoxic T cells to the sites of tumors.23 The importance of α-GalCer and PD-1 blockade on CD8+ T cell cytotoxic activity has been demonstrated by Bae et al.,24 who found that administration of an iNKT agonist in an anti-PD-1-resistant tumor
model re-stimulated exhausted CD8+ T cells through the enhanced secretion of IL-2 and IL-12. They also observed a synergistic
increase in the antitumor effect between α-GalCer-loaded APCs and PD-1 blockade. Moreover,
in vitro assays with human peripheral blood mononuclear cells (PBMCs) showed that the simultaneous
co-administration of an anti-PD-L1 antibody and α-GalCer-pulsed APCs enhanced both
the direct cytotoxic and indirect TH1 cytokine release functions of iNKT cells, enhancing
their antitumor immunostimulatory functions.25 Lastly, preclinical studies in mice have confirmed the similarities between ABX196
and α-GalCer concerning in vitro and in vivo stimulation of iNKT cells.15 Toxicity reports from these same experiments suggested no considerable adverse effects
of ABX196 in mice and monkeys at the doses necessary for immune activation, although
hepatic toxicity in the form of elevated transaminases was observed in mice at doses
higher than that required for immune activation.15
Clinical Data: ABX196 and Nivolumab in the Treatment of Hepatocellular Carcinoma
Clinical studies assessing the immunotherapeutic rationale of administering iNKT agonists
in combination with ICIs are sparse and have only recently been initiated for the
treatment of hepatocellular carcinoma (HCC). HCC nearly always develops secondary
to chronic liver inflammation, as this produces an immunosuppressive microenvironment
that accommodates immune cell exhaustion.26 Exhausted immune cells exist in an inactive anergic state, expressing high levels
of inhibitory co-receptors, such as PD-1 and CTLA-4, and low levels of effector cytokines.
As a result, anti-PD-1 antibodies have been studied in HCC and found to be an effective
treatment.27 Nivolumab received FDA approval in September 2017 for patients with advanced HCC
previously treated with the multi-kinase inhibitor sorafenib during the phase I/II
dose-escalation and dose-expansion CheckMate-040 study.28 Nivolumab resulted in significant tumor diminution compared to first-line sorafenib
therapy; however, its objective response rate (ORR) remained low at 15% (95% CI 6-28)
in the dose-escalation phase and 20% (95% CI 15-26) in the dose-expansion phase. This
has prompted additional efforts to improve nivolumab response rates in HCC treatment,
and the first open label, uncontrolled phase I/II clinical trial to assess combination
therapy of ABX196 with nivolumab is now underway at the Scripps M.D. Anderson Cancer
Center [NCT03897543]. Importantly, the trial addresses the question of whether the
immunostimulatory effects of ABX196 may help bolster the efficacy of nivolumab immunotherapy
in HCC by specifically targeting and reversing iNKT cell anergy.
Since Hepatitis B infection is associated with increased risk of HCC, the adjuvant
activity of ABX196 may play a critical role in HCC immunotherapeutic control. In a
phase I first-in-human dose-escalation study, ABX196 induced a strong anti-HepB antibody
response when used as an adjuvant for a prophylactic hepatitis B vaccine.15 In all forty-four healthy male subjects treated, ABX196 elicited a stimulation of
NKT cells in vivo as demonstrated by the downregulation of NKT TCR and pronounced antibody response.
Adverse side effects were mild to moderate and associated with elevated IFN-γ levels,
consistent with acute activation of hepatic iNKT cells by ABX196. Similarly, many
clinical trials (Table 1) have tested the anticancer therapeutic potential of α-GalCer in humans, and it has
been shown to be a safe and well-tolerated treatment plan, although its effectiveness
in these trials was limited due to iNKT cell anergy and the development of immunosuppressive
tumor microenvironments.42
Table 1.
Clinical Trials Evaluating α-GalCer-Mediated Stimulation of iNKT Cells
| Clinical trial |
No. of participants |
Treatment |
Result |
Cancer type(s) |
| Giaccone et al.29 |
24 |
α-GalCer; intravenous |
7 SD |
Solid tumors |
| Nieda et al.30 |
12 |
α-GalCer-pulsed CD1d-expressing DCs; intravenous |
3 reductions in tumor markers/mass |
Solid tumors |
| Ishikawa et al.31 |
11 enrolled, 9 completed |
α-GalCer-pulsed DCs; intravenous |
5 SD |
NSCLC |
| Chang et al.32 |
6 enrolled, 5 completed |
α-GalCer loaded onto monocyte-derived mature DCs; intravenous |
4 reductions in tumor markers or SD |
MM, RCC, Anal SCC |
| Motohashi et al.33 |
6 |
α-GalCer-activated Vα24 iNKT cells; intravenous |
4 SD |
NSCLC |
| Uchida et al.34 |
9 |
α-GalCer-pulsed APCs; administered in nasal submucosa |
1 PR, 5 SD |
HNSCC |
| Motohashi et al.35 |
23 enrolled, 17 completed |
α-GalCer-pulsed PBMC cultured with IL-2 and GM-CSF; intravenous |
5 SD |
NSCLC |
| Kunii et al.36 |
8 |
Intra-arterial infusions of α-GalCer-activated Vα24 iNKT cells + submucosal injections
of α-GalCer-pulsed APCs
|
3 PR, 4 SD |
HNSCC |
| Kurosaki et al.37 |
17 |
α-GalCer-pulsed APC injections into nasal or oral floor submucosa |
Increased levels of iNKT cells/IFN-γ |
HNSCC |
| Yamasaki et al.38 |
10 |
Nasal submucosal administration of α-GalCer-pulsed APCs
+ intra-arterial infusion of α-GalCer-activated iNKT cells via tumor-feeding arteries |
5 PR, 5 SD |
HNSCC |
| Nicol et al.39 |
12 |
α-GalCer-pulsed DCs; 2 treatments intravenous, 2 treatments intradermal |
3 PR, 3 SD |
Solid tumors |
| Nagato et al.40 |
4 |
α-GalCer-pulsed APCs; intravenous |
Increased levels of iNKT cells in TILs, increased IFN-γ levels |
NSCLC |
| Richter et al.41 |
6 |
α-GalCer-loaded monocyte-derived DCs + low-dose lenalidomide; intravenous |
3 reductions in tumor-associated monoclonal immunoglobulin |
Asymptomatic myeloma |
Abbreviations: SD, stable disease; PR, partial remission; HNSCC, head and neck squamous cell carcinoma;
NSCLC, non-small cell lung cancer; MM, multiple myeloma; RCC, renal cell carcinoma;
SCC, squamous cell carcinoma; DC, dendritic cell; APC, antigen presenting cell; TIL,
tumor infiltrating lymphocyte.
CAR-T Cells in Combination with PD-1 Blockade
Background: The Therapeutic Evolution of CAR-T Cells
Chimeric antigen receptor (CAR)-T cells are genetically modified T cells designed
to express a synthetic TCR for use in anticancer immunotherapy. T cells are isolated
from human blood and engineered to express a unique CAR. CAR-T cells are then stimulated
to expand ex vivo and are infused back into the patient to kill tumor cells expressing the corresponding
CAR-T cell antigen.43 CAR constructs are hybrid molecules consisting of three regions: i) the extracellular
ectodomain, usually composed of a single chain variable fragment (scFv) obtained from
a tumor antigen-reactive antibody, ii) the transmembrane domain to support CAR stability,
and iii) the intracellular endodomain, composed of signaling peptides responsible
for cell activation and co-stimulation following tumor antigen recognition (Figure 2).44 CAR-T cells are separated into four generations based on the composition of their
endodomains.45 First generation CARs contain a scFv attached to a CD3ζ-derived intracellular signaling
molecule, the primary transmitter of endogenous TCR stimulation. Second generation
CARs contain an additional co-stimulatory molecule as part of the signal transduction
region.46 Third generation CARs combine multiple intracellular co-stimulatory domains to increase
cytokine production. Fourth generation CARs, also referred to as T cell redirected
for universal cytokine-mediated killing (TRUCKs), contain a transgenic load of immune
modifiers, such as cytokines, co-stimulatory ligands and enzymes, that upon release
help activate and recruit innate immune cells to eliminate antigen-negative tumor
cells.47 TRUCKs enhance antitumoral activity through inducible IL-12, creating an immunostimulatory
tumor microenvironment and favorably redirecting host lymphocytes toward the tumor
site.48
Figure 2
Structure of the Chimeric Antigen Receptor (CAR)-T cell
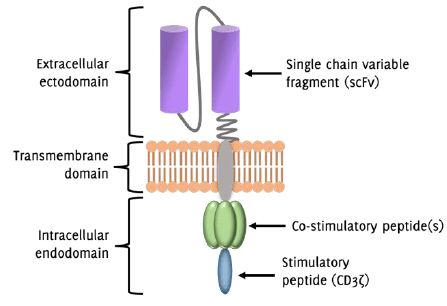
Legend: Simplified diagram of the chimeric antigen receptor (CAR)-T cell structure. The extracellular
ectodomain communicates with a specific tumor cell antigen via the single chain variable
fragment (scFv), which is derived from an antibody that reacts with a given tumor
antigen. The transmembrane domain provides stability to the CAR structure. The endodomain
is responsible for communicating intracellular signals that promote T cell activation.
Rationale for CAR-T Cell Combination Therapy with ICIs
While conventional CAR-T cell therapy has demonstrated clinical success against B
cell hematologic malignancies,49,50 its efficacy is limited by several important obstacles, including high toxicity,
immunosuppressive tumor milieu, and CAR-T cell dysfunction.51 One of the primary reasons for poor treatment response and relapse after CAR-T cell
therapy is inefficient T cell expansion and a lack of persistent T cell activation
following infusion of CAR-T cells into patients.44 It is thought that CAR-T cell dysfunction and non-persistence is driven by co-inhibitory
pathways induced by checkpoint blockade that lead to T cell anergy.52 CAR-T cells were shown to upregulate immune checkpoint receptors, such as PD-1, CTLA-4
and lymphocyte activating gene-3 (LAG-3), in patients with chronic lymphocytic leukemia
(CLL) unresponsive to anti-CD19 CAR-T cell therapy.53 CLL is a hematologic malignancy with particularly poor response rates to CAR-T cell
therapy and is known to facilitate an immunosuppressive, pro-tumor microenvironment.54 PD-L1 expression was found to be significantly higher in 112 CLL patients than in
non-CLL controls.55 Similarly, mesothelin-specific CAR TILs (tumor-infiltrating lymphocytes) administered
to mice bearing human mesothelin-expressing flank tumors underwent rapid and spontaneous
loss of functional activity associated with increased expression of the surface inhibitory
receptors PD-1, LAG3, T cell immunoglobulin- and mucin-domain-containing molecule
3 (TIM3) and 2B4 (CD244).56 The aberrant expression of inhibitory molecules has been demonstrated in CAR-T cell
clinical trials as well.57 Infusion of anti-CD19 CAR-T cells to patients with advanced B cell lymphomas resulted
in at least three-fold increase in expression of PD-1 at the surfaces of CD4+ CAR-positive cells in 8 out of 11 patients.57 These studies suggest that checkpoint-based immunosuppression is an important mechanism
mediating tumor resistance to CAR-T cell therapy. Therefore, strategies that block
inhibitory immune checkpoint pathways in combination with CAR-T cell therapy possess
powerful immunotherapeutic potential.
Preclinical Data: CAR-T Cells with PD-1 Blockade
CAR-T cell combination therapy with PD-1 blockade has demonstrated improved antitumor
effects in multiple preclinical models. In adoptive transfer studies of mice bearing
human epidermal growth factor receptor-2 (HER-2)+ tumors, anti-PD-1 antibodies enhanced HER-2-specific CAR-T cell functionality, significantly
increased markers of activation and proliferation, improved tumor growth inhibition,
and reduced the percentage of myeloid-derived suppressor cells, which – when produced
in excess – are known to aid in tumor metastasis and immune evasion.58 Similarly, PD-L1 inhibition in mouse CLL models reactivated immune effector functions
and restored cytotoxic CD8+ T cell activity as well as immune synapse formation ex vivo and in vivo by preventing exhaustion-like T cell phenotypes.59 Experiments conducted with an orthotopic mouse model of pleural mesothelioma showed
that PD-1 pathway interference restored the effector function of exhausted CD28-specific
CAR-T cells.60 Gargett et al.61 demonstrated that third generation GD2-specific CAR-T cells would undergo significant
activation-induced cell death (AICD) after repeated antigen stimulation in vitro; however, PD-1 blockade enhanced both CAR-T cell survival and promoted killing of
PD-L1+ tumor cell lines. CRISPR-Cas9-mediated editing of CAR-T cells, which rendered them
non-responsive to PD-1 signaling, improved antitumor CAR-T cell activity both in vitro and in vivo.62 Finally, Hui et al. 63 showed that PD-1/PD-L1 interactions suppressed CAR-T cell activity by blocking CD28
signaling, suggesting that upregulation of costimulatory pathways is an important
mechanistic response of CAR-T cells to anti-PD-1/PD-L1 therapy.
Clinical Data: CAR-T Cells with PD-1 Blockade
Clinical trials employing CAR-T cell combination therapy with PD-1 blockade have already
shown promising results. In a single-institution study at the Children's Hospital
of Philadelphia, fourteen pediatric patients with heavily treated, relapse B cell
acute lymphoblastic leukemia (B-ALL) and poor responses to CAR-T cell therapy were
treated with CD19-specific CAR-T cell therapy in combination with an anti-PD-1 monoclonal
antibody.64 Encouraging results were particularly observed in patients with early B-cell recovery
and bulky extramedullary disease. Three of 6 patients treated with PD-1 inhibitor
and CAR-T cells for early B cell recovery reestablished B cell aplasia, an indication
of persistent CAR-T cell activation. In a cohort of four patients treated with pembrolizumab
and CAR-T cells for extramedullary disease, 2 partial remissions (PRs) and 2 complete
remissions (CRs) were seen. However, in the 4 remaining patients who were unsuccessful
in achieving remission with initial CAR-T cell therapy, only PRs were observed with
CAR-T cell and pembrolizumab combination therapy. Adverse effects of combination therapy
included fever, acute pancreatitis, hypothyroidism, joint pains, as well as moderate
to severe pancytopenia. The study supports the hypotheses that upregulation of the
PD-1/PD-L1 signaling axis may be a driving force in the development of resistance
to CAR-T cell immunotherapy. The study also suggests that ICI combination therapy
at the time of CAR-T cell administration may be a safe and durable strategy for preventing
subsequent AICD in the treatment of B-ALL.
A similar single-institution trial at the Abramson Cancer Center of the University
of Pennsylvania attempted to evaluate the role of pembrolizumab as salvage therapy
for patients who experienced worsening disease following initial CAR-T cell infusion.65 The study enrolled 12 patients with progressive or relapse B cell non-Hodgkin lymphomas
with partial or no response to CD19-specific CAR-T cell therapy. Pembrolizumab was
administered to these patients every 3 weeks until disease progression or adverse
toxic side effects were observed. The addition of PD-1 blockade after prior ineffective
anti-CD19 CAR-T cell therapy produced an ORR of 27% (1 CR and 2 PRs), including 1
patient with stable disease and 7 patients with progressive disease. Nine of 12 patients
demonstrated a re-expansion of peripheral blood CAR-T cells after the first pembrolizumab
dose, although this cellular re-expansion did not correlate with clinical outcome.
Nevertheless, this study highlights the key theory that ICIs may reinvigorate exhausted
CAR-T cells in patients with poor or failed responses to initial CAR-T cell therapy,
and further studies should be explored to translate this CAR-T cell re-expansion into
clinically efficacious use. At the moment, several clinical trials (Table 2) are attempting to address the optimal timing of administration, dosing, efficacy,
and safety of CAR-T cell combination therapy with ICIs, particularly in patients who
have failed first-line therapies with relapsed or refractory progression of their
cancers. These trials address ICI and CAR-T cell combination therapy in two primary
treatment scenarios: 1) when the agents are administered simultaneously, or 2) when
the ICI is administered for a limited duration shortly after CAR-T cell infusion.
Taken together, these studies attempt to assess both the potential of concurrent ICI
and CAR-T cell therapy to prevent the development of future AICD as well as the salvage
potential of ICIs to enhance prior ineffective CAR-T cell therapy. Investigating ICI
and CAR-T cell combination therapy from both of these angles may provide a better
understanding of the appropriate timeframe in which ICIs can most effectively enhance
and prevent resistance to CAR-T cell therapy.
Table 2.
Clinical Trials Evaluating CAR-T Cell Combination Therapy With PD-1 Blockade
| Clinical trial identifier |
Sponsor/study name |
Patients enrolled |
CAR-T |
ICI |
Cancer type(s) |
| NCT03310619 |
Celgene/PLATFORM |
100 |
JCAR017 |
Durvalumab |
NHL, DLBCL, FL |
| NCT03287817 |
Autolus Limited/ALEXANDER |
120 |
AUTO3 |
Pembrolizumab |
DLBCL |
| NCT02706405 |
Fred Hutchinson Cancer Research Center |
42 |
JCAR014 |
Durvalumab |
NHLs + gene rearrangement(s), DLBCL, PMBL |
| NCT03630159 |
Novartis Pharmaceuticals/PORTIA |
32 |
CTL019 |
Pembrolizumab |
DLBCL |
| NCT02926833 |
Kite, A Gilead Company/ZUMA-6 |
37 |
KTE-C19 |
Atezolizumab |
DLBCL |
| NCT03726515 |
University of Pennsylvania |
7 |
CART-EGFRvIII |
Pembrolizumab |
Glioblastoma |
| NCT04003649 |
City of Hope Medical Center |
60 |
IL13Ralpha2-CRT T cells |
Ipilimumab, nivolumab |
Glioblastoma |
| NCT03525782 |
The First Affiliated Hospital of Guangdong Pharmaceutical University |
60 |
Anti-MUC1 CAR-T cells |
Nivolumab |
NSCLC |
| NCT02414269 |
Memorial Sloan Kettering Cancer Center |
66 |
iCasp9M28z, T cells |
Pembrolizumab |
Lung/breast cancers, mesotheliom |
Legend: NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma;
PMBL, primary mediastinal B cell lymphoma; NSCLC, non-small cell lung cancer
NK Cells in Combination with PD-1 Blockade
Applications, Advantages, and Challenges of NK Cell Therapy
Natural killer (NK) cells are cytotoxic innate lymphoid cells that play a vital role
in antitumor immunity due to their unique ability to detect and eliminate malignant
cells with downregulated surface expression of self-MHC-I molecules.66 NK cell functions vary widely and include degranulation, cytokine secretion primarily
in the form of IFN-γ, and direct cytotoxicity due to an elaborate interaction of inhibitory
and activating signals.67 Many antitumor therapeutic strategies have emerged in recent years that utilize the
cytotoxic and immunoregulatory activities of NK cells. Adoptive transfer therapy,
in which NK cells from a healthy donor are isolated, activated ex vivo in an IL-2 or IL-15 solution, then infused into cancer patients, has proven to be
an effective and nontoxic antitumor treatment.68 More recently, isolated NK cells have been genetically modified to express a unique
tumor-antigen CAR prior to re-infusion. CD19- and CD20-specific CAR-NK cells have
shown successful preclinical tumor growth inhibition in a variety of B cell malignancies.69 CAR-NK cells targeting HER-2, epidermal growth factor receptor (EGFR), natural killer
group 2D (NKG2D), and disialoganglioside GD2 receptors, all of which are overexpressed
in tumor cells, have also shown preclinical antitumor efficacy against solid tumors.69 CAR-NK cell therapy has advantages over CAR-T cell therapy in that it does not induce
graft versus host disease (GVHD),70,71 nor does the CAR modification prevent the NK cell from carrying out its non-specific
innate functions, thus limiting the occurrence of antigen loss and tumor escape.72 In addition, CAR-NK cell therapy eliminates the need for a personalized, autologous
product typically required with CAR-T cell therapy. CAR-NK cell therapy, therefore,
has the potential to be an affordable and readily available, “off-the-shelf” treatment
option. The aforementioned therapeutic benefits are limited in part by the ability
of injected NK cells to migrate to tumor sites, persist, and expand in vivo.73 NK cells express a range of inhibitory receptors, including PD-1, PD-L1, CTLA-4,
T cell immunoglobulin and mucin-containing protein 3 (TIM3), T cell immunoreceptor
with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT),
LAG-3, interleukin-1 receptor 8 (IL-1R8), and CD96, in addition to more well-established
inhibitory receptors, like killer-cell immunoglobulin-like receptor (KIR) and the
C-type lectin inhibitory receptor CD94/natural killer group 2A (NKG2A).74,75 Research into the blockade of these inhibitory NK cell immune checkpoint pathways
is ongoing and early data reflect an encouraging possibility of immune checkpoint
inhibition to overcome the immunosuppressive limitations currently associated with
NK cell therapy.76 For the purposes of this review, literature pertaining specifically to inhibition
of the PD-1/PD-L1 axis was the focus.
Rationale for Combining NK Cell Therapy with PD-1 Blockade
PD-1 is highly expressed on a distinct subpopulation of NK cells with impaired immunostimulatory
capabilities that are detectable in approximately 25% of healthy people.77 NK cell populations with high PD-1 expression demonstrate significantly reduced function
and are found in greater proportion in patients with ovarian carcinoma,77 Kaposi sarcoma,78 multiple myeloma,79 and head and neck cancers.80 In vitro studies have confirmed that PD-1 receptors become upregulated at the surface of healthy
control NK cells upon extended contact with activating ligands,78 suggesting that PD-1 helps induce, as it does for T lymphocytes, NK cell anergy.
This increased expression of PD-1 at the surface of NK cells correlates with poorer
survival prognosis in esophageal and liver cancers.81 More recent studies have determined that upregulated PD-L1 expression at the surface
of NK cells also mediates exhaustive NK phenotypes. Dong et al., 75 for instance, discovered that some tumors can induce PD-L1 expression on NK cells
via protein kinase B (AKT) signaling. These discoveries support the idea that NK cells
are a valuable target in immunotherapeutic approaches that inhibit PD-1/PD-L1 interactions,
especially when these ICIs are used to treat tumors that are MHC-I deficient. Thus,
utilizing PD-1 blockade may be an excellent additive strategy for immunotherapy regimens
that harness the antitumor capabilities of NK cells.
Preclinical data: NK cells with PD-1 blockade
Preclinical studies have shown that inhibiting PD-1 and PD-L1 checkpoint receptors
enhances the immunotherapeutic efficacy of NK cells. Benson et al.79 demonstrated that a PD-1 blocking antibody improved human NK cell functionality against
autologous, primary multiple myeloma cells in vitro through a mechanism involving NK cell trafficking, immune complex formation, and
enhanced cytotoxicity directed toward PD-L1+ tumor cells. Blocking PD-1/PD-L1 signaling notably enhanced cytokine secretion and
inhibited NK cell apoptosis in vitro. Importantly, administration of an anti-PD-1 antibody significantly slowed tumor
growth in HCC xenografts, and this beneficial antitumor response was diminished by
NK cell depletion, indicating an NK-dependent antitumor mechanism in response to PD-1
blockade.81 Hsu et al. 82 performed similar experiments on several mouse models of cancer, including lymphoma,
melanoma, prostate adenocarcinoma, and colon carcinoma, and determined that the release
of PD-1-imposed inhibition activated an NK response that was indispensable for the
full effect of ICI immunotherapy. Oyer et al. 83 observed a significant improvement in NK cell antitumor efficacy, persistence, and
retention of cytotoxic activity in mouse ovarian cancer models when combined with
anti-PD-L1 antibody. The group showed that expanded NK cells secreted large amounts
of IFN-γ, which induced expression of PD-L1 on human ovarian cancer cells in vivo. These findings support NK cell combination therapy with anti-PD-L1 antibody, irrespective
of initial tumor PD-L1 status. Lastly, Dong et al. 75 determined that various PD-L1- tumor cell lines still responded favorably to anti-PD-L1 monoclonal antibody therapy,
because the anti-PD-L1 antibody directly targeted and activated PD-L1+ NK cells in a PD-1 independent process.
Clinical Data: NK Cells with PD-1 Blockade
While preclinical data has confirmed the immunostimulatory advantage of PD-1 blockade
on NK cell functionality, clinical trials are new and in their early stages. A phase
II study assessing the effects of pembrolizumab on NK cell exhaustion in patients
with malignant melanoma was recently terminated due to enrollment difficulties [NCT03241927].
Nevertheless, several clinical trials (Table 3) evaluating the therapeutic benefit of PD-1 blockade on NK cell antitumor activity
are currently underway.
Table 3.
Clinical Trials Evaluating NK Cell Combination Therapy With PD-1 Blockade.
| Clinical trial identifier |
Sponsor |
Patients enrolled |
ICI + NK |
Cancer type(s) |
| NCT03958097 |
First Hospital of Jilin University |
20 |
Sintilimab (anti-PD-1) + NK cells (autologous NK cells; collected by apheresis) |
NSCLC |
| NCT03815084 |
Allife Medical Science and Technology Co., Ltd. |
100 |
Pembrolizumab + DC-NK |
Solid tumors |
| NCT03937895 |
SMT bio Co., Ltd. |
40 |
Pembrolizumab + SMT-NK (allogeneic NK cells) |
BTC |
Legend: NSCLC, non-small cell lung cancer; BTC, biliary tract cancer
Conclusion
Immunotherapy continues to revolutionize cancer treatment in the twenty-first century.
ABX196, CAR-T cell and NK cell immunotherapies, in particular, have shown compelling
preclinical data and are in early phase studies to determine if this activity can
be translated into patient care. These therapeutics have mechanisms of action that
are distinct from approved ICIs, which may overcome some of the limitations that have
plagued ICI immunotherapy. Modulating multiple levers of the immune system simultaneously
by co-administering ICIs with either ABX196, CAR-T cells or NK cells may further the
essential, as-yet unmet, goal of overcoming tumor resistance to ICI immunotherapy.
While awaiting the results of these agents' early clinical trials, additional studies
should be pursued to both enhance and optimize these promising new immunotherapy approaches.
Acknowledgments
None
Conflict of Interest Statement & Funding
Darren S. Sigal is an advisor for Celularity, Molecular Stethoscope, Curematch, and
DrugCendR, has a patent on a method of use of TRK inhibitors in neuroendocrine tumors,
and is on the speaker bureau for Celgene and Bayer. Jonathan A. Hermel and Cassi M.
Bruni have no conflicts to declare. This research received no external funding.
Author Contributions
Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Project
Administration, Resources, Supervision, Validation: JAH, DSS. Visualization: JAH,
DSS, CMB. Writing – Original Draft Preparation: JAH, DSS, Writing – Review & Editing:
JAH, DSS, CMB.
References
1.
Madden K, Kasler MK. Immune Checkpoint Inhibitors in Lung Cancer and Melanoma. Semin Oncol Nurs. 2019 Oct;35(5):150932.
2.
Herrscher H, Robert C. Immune checkpoint inhibitors in melanoma in the metastatic, neoadjuvant, and adjuvant
setting. Curr Opin Oncol. 2020 Mar;32(2):106-13.
3.
Ren S, Wang C, Shen J, Zhu C. Neoadjuvant immunotherapy with resectable non-small cell lung cancer: recent advances
and future challenges. J Thorac Dis. 2020 Apr;12(4):1615-20.
4.
Sambi M, Bagheri L, Szewczuk MR. Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy
and Patient Response Rates. J Oncol. 2019 Feb 28;2019:4508794.
5.
Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019 Mar;19(3):133-50.
6.
Nair S, Dhodapkar MV. Natural Killer T Cells in Cancer Immunotherapy. Front Immunol. 2017 Sep 22;8:1178.
7.
Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility
complex class I-specific CD4+ and CD4-8-T cells in mice and humans. J Exp Med. 1994 Sep 1;180(3):1097-106.
8.
Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995 May 12;268(5212):863-5.
9.
Zajonc DM, Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr Opin Struct Biol. 2007 Oct;17(5):521-9.
10.
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877-900.
11.
McEwen-Smith, RM, Salio M, Cerundolo V. The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunol Res. 2015 May;3(5):425-35.
12.
Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005 Sep;115(9):2572-83.
13.
Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, et al. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in
vivo. Proc Natl Acad Sci USA. 2003 July 8;100(14):8395-400.
14.
Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002 Apr;14(2):165-71.
15.
Tefit JN, Crabe S, Orlandini B, Nell H, Bendelac A, Deng S, et al. Efficacy of ABX196, a new NKT agonist, in prophylactic human vaccination. Vaccine. 2014 Oct 21;32(46):6138-45.
16.
Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005 Sep;115(9):2572-83.
17.
Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, et al. The response of natural killer T cells to glycolipid antigens is characterized by
surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003 Sep 16;100(19):10913-8.
18.
Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, et al. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the
induction and maintenance of invariant NKT cell anergy. J Immunol. 2008 Nov 15;181(10):6707-10.
19.
Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities
of glycolipid-activated invariant NKT cells. J Immunol. 2009 Mar 1;182(5):2816-26.
20.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
21.
Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines
with the Level of Evidence. Cancers (Basel). 2020 Mar 20;12(3):738.
22.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism,
Combinations, and Clinical Outcome. Front Pharmacol. 2017 Aug 23;8:561.
23.
Durgan K, Ali M, Warner P, Latchman YE. Targeting NKT cells and PD-L1 pathway results in augmented anti-tumor responses in
a melanoma model. Cancer Immunol Immunother. 2011 Apr;60(4):547-58.
24.
Bae EA, Seo H, Kim BS, Choi J, Jeon I, Shin KS, et al. Activation of NKT Cells in an Anti-PD-1-Resistant Tumor Model Enhances Antitumor Immunity
by Reinvigorating Exhausted CD8 T Cells. Cancer Res. 2018 Sep 15;78(18):5315-26.
25.
Kamata T, Suzuki A, Mise N, Ihara F, Takami M, Makita Y, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor
immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016 Dec;65(12):1477-89.
26.
Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer. 2016 Feb 1;122(3):367-77.
27.
Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020 Apr;39(18):3620-37.
28.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label,
non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017 Jun 24;389(10088):2492-502.
29.
Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000)
in patients with solid tumors. Clin Cancer Res. 2002 Dec;8(12):3702-9.
30.
Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in
highly coordinated secondary activation of acquired and innate immunity. Blood. 2004 Jan 15;103(2):383-9.
31.
Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients
with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005 Mar 1;11(5):1910-7.
32.
Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide
loaded mature dendritic cells in cancer patients. J Exp Med. 2005 May 2;201(9):1503-17.
33.
Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced
and recurrent non-small cell lung cancer. Clin Cancer Res. 2006 Oct 15;12,(20 Pt 1):6079-86.
34.
Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration
to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008 Mar;57(3):337-45.
35.
Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral
blood mononuclear cells in patients with advanced and recurrent non-small cell lung
cancer. J Immunol. 2009 Feb 15;182(4):2492-501.
36.
Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed
antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009 Jun;100(6):1092-8.
37.
Kurosaki M, Horiguchi S, Yamasaki K, Uchida Y, Motohashi S, Nakayama T, et al. Migration and immunological reaction after the administration of alphaGalCer-pulsed
antigen-presenting cells into the submucosa of patients with head and neck cancer. Cancer Immunol Immunother. 2011 Feb;60(2):207-15.
38.
Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted
adoptive immunotherapy. Clin Immunol. 2011 Mar;138(3):255-65.
39.
Nicol AJ, Tazbirkova A, Nieda M. Comparison of clinical and immunological effects of intravenous and intradermal administration
of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin Cancer Res. 2011 Aug 1;17(15):5140-51.
40.
Nagato K, Motohashi S, Ishibashi F, Okita K, Yamasaki K, Moriya Y, et al. Accumulation of activated invariant natural killer T cells in the tumor microenvironment
after alpha-galactosylceramide-pulsed antigen presenting cells. J Clin Immunol. 2012 Oct;32(5):1071-81.
41.
Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting
human NKT cells in myeloma. Blood. 2013 Jan 17;121(3):423-30.
42.
Zhang Y, Springfield R, Chen S, Li X, Feng X, Moshirian R, et al. α-GalCer and iNKT Cell-Based Cancer Immunotherapy: Realizing the Therapeutic Potentials. Front Immunol. 2019 Jun 6;10:1126.
43.
Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020 Mar;21(3):e168-78.
44.
Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol. 2019 Jun 11;12 (1):59.
45.
Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017 Jun 24;5:22.
46.
Guedan S, Posey AD, Jr, Shaw C, Wing A, Da T, Patel PR, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018 Jan 11;3(1):e96976.
47.
Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015 May;15(8):1145-54.
48.
Kueberuwa G, Kalaitsidou M, Cheadle E, Hawkins RE, Gilham DE. CD19 CAR T Cells Expressing IL-12 Eradicate Lymphoma in Fully Lymphoreplete Mice through
Induction of Host Immunity. Mol Ther Oncolytics. 2018 Mar 30;8:41-51.
49.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell
lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019 Jan;20(1):31-42.
50.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Investigators, J., Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019 Jan 3;380(1):45-56.
51.
D'Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 2018 Feb 15;9(3):282.
52.
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy
in vivo. Nat Biotechnol. 2018 Oct;36(9):847-56.
53.
Galon J, Rossi J, Turcan S, Danan C, Locke FL, Neelapu SS, et al. Characterization of anti-CD19 chimeric antigen receptor (CAR) T cell-mediated tumor
microenvironment immune gene profile in a multicenter trial (ZUMA-1) with axicabtagene
ciloleucel (axi-cel, KTE-C19). J Clin Oncol. 2017 May 30;35(Suppl 15):3025.
54.
Bair SM, Porter DL. Accelerating chimeric antigen receptor therapy in chronic lymphocytic leukemia: The
development and challenges of chimeric antigen receptor T-cell therapy for chronic
lymphocytic leukemia. Am J Hematol. 2019 May;94(S1):S10-7.
55.
Grzywnowicz M, Karczmarczyk A, Skorka K, Zajac M, Zaleska J, Chocholska S, et al. Expression of Programmed Death 1 Ligand in Different Compartments of Chronic Lymphocytic
Leukemia. Acta Haematol. 2015 Nov;134(4):255-62.
56.
Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric
antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014 Aug 15;20(16):4262-73.
57.
Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies
can be effectively treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 2015 Feb 20;33(6):540-9.
58.
John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors
by gene-modified T cells. Clin Cancer Res. 2013 Oct 15;19(20):5636-46.
59.
McClanahan F, Hanna B, Miller S, Clear AJ, Lichter P, Gribben JG, et al. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in
a mouse model of chronic lymphocytic leukemia. Blood. 2015 July 9;126(2):203-11.
60.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated
inhibition. J Clin Invest. 2016 Aug 1;126(8):3130-44.
61.
Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, et al. GD2-specific CAR T Cells Undergo Potent Activation and Deletion Following Antigen
Encounter but can be Protected From Activation-induced Cell Death by PD-1 Blockade. Mol Ther. 2016 Mar;24(6):1135-49.
62.
Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017 Jan;27(1):154-7.
63.
Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017 Mar 31;355(6332):1428-33.
64.
Li AM, Hucks GE, Dinofia AM, Seif AE, Teachey DT, Baniewicz D, et al. Checkpoint Inhibitors Augment CD19-Directed Chimeric Antigen Receptor (CAR) T Cell
Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood. 2018 Nov 29;132(Suppl 1):556.
65.
Chong EA, Svoboda J, Dwivedy Nasta S, Landsburg DJ, Winchell N, Napier E, et al. Sequential Anti-CD19 Directed Chimeric Antigen Receptor Modified T-Cell Therapy (CART19)
and PD-1 Blockade with Pembrolizumab in Patients with Relapsed or Refractory B-Cell
Non-Hodgkin Lymphomas. Blood. 2018 Nov 29;132(Suppl 1):4198.
66.
Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol. 2018 Aug 13;9:1869.
67.
Paul S, Lal G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer
Immunotherapy. Front Immunol. 2017 Sep 13;8:1124.
68.
Rohaan MW, Wilgenhof S, Haanen JBAG. Adoptive cellular therapies: the current landscape. Virchows Arch. 2019 Apr;474(4):449-61.
69.
Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017 Aug 2;25(8):1769-81.
70.
Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk leukemia. Semin Immunol. 2014 Apr;26(2):173-9.
71.
Rezvani K, Rouce RH. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 2015 Nov 17;6:578.
72.
Mehta RS, Rezvani K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of
Cancer. Front Immunol. 2018 Feb 15;9:283.
73.
Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid
tumors. Front Immunol. 2015 Apr 29;6:202.
74.
Kwon HJ, Kim N, Kim HS. Molecular checkpoints controlling natural killer cell activation and their modulation
for cancer immunotherapy. Exp Mol Med. 2017 Mar 31;49(3):e311.
75.
Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1 negative tumors identifies
NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019 Oct;9(10):1422-37.
76.
Khan M, Arooj S, Wang H. NK Cell-Based Immune Checkpoint Inhibition. Front Immunol. 2020 Feb 13;11:167.
77.
Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, et al. Identification of a subset of human natural killer cells expressing high levels of
programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol. 2017 Jan;139(1):335-6.e3.
78.
Beldi-Ferchiou A, Lambert M, Dogniaux S, Vely F, Vivier E, Olive D, et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi
sarcoma. Oncotarget. 2016 Nov 8;7(45):72961-77.
79.
Benson DM, Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect:
a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010 Sep 30;116(13):2286-94.
80.
Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD-L1 Mediates Dysfunction in Activated PD-1(+) NK Cells in Head and Neck Cancer Patients. Cancer Immunol Res. 2018 Dec;6(12):1548-60.
81.
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li, C, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated
anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017 Nov 2;36(44):6143-53.
82.
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018 Oct 1;128(10):4654-68.
83.
Oyer JL, Gitto SB, Altomare DA, Copik AJ. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology. 2018 Aug 27;7(11):e1509819.
Jonathan A. Hermel, 1 BA, Tulane University School of Medicine, Department of Graduate Medical Education,
United States
Cassi M. Bruni, 2 MS, University of California, San Diego, Department of Cellular and Molecular Medicine,
United States
Darren S. Sigal, 3 MD, Scripps Clinical Medical Group, Division of Hematology/Oncology, United States
About the Author: Jonathan A. Hermel is a third-year medical student at Tulane University School of
Medicine in New Orleans, Louisiana. Darren S. Sigal is a hematologist/oncologist at
Scripps Clinic in La Jolla, California, where he is head of the gastrointestinal cancer
division. He is an active participant and developer of on-going clinical studies focused
on the treatment of pancreatic cancer, colorectal cancer, and liver cancer. Cassi
M. Bruni is a recent graduate with honors of the M.S. Biology program at the University
of California, San Diego, Department of Cellular and Molecular Medicine.
Correspondence: Darren S. Sigal. Address: 10710 N Torrey Pines Rd, La Jolla, CA 92037, United States.
Email: Sigal.Darren@scrippshealth.org
Editor: Mihnea-Alexandru Găman & Francisco J. Bonilla-Escobar
Student Editors: Benjamin Liu
Copyeditor: Ciara Egan
Proofreader: Sohaib Haseeb
Layout Editor: Judie Joo
Cite as: Hermel JA, Bruni CM, Sigal DS. Novel Combination Strategies to Enhance Immune Checkpoint Inhibition in Cancer Immunotherapy: A Narrative Review. Int J Med Students. 2020 Sep-Dec;8(3):273-80.
Copyright © 2020 Jonathan A. Hermel, Cassi M. Bruni, Darren S. Sigal
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 8, NUMBER 3, December 2020

