Case Report
A Cadaveric Study on the Anomalous Origin of Renal and Gonadal Vasculature: An Observational
Study
Penelope C. Farris1, Dylan M. Macciola2, Lauren N. Barazani3, Justin R. Nathan4, Danielle Quinn5, Daniel F. Peters6
doi: http://dx.doi.org/10.5195/ijms.2022.757
Volume 10, Number 3: 316-320
Received 21 09 2020;
Rev-request 28 10 2020;
Rev-request 09 04 2021;
Rev-request 16 03 2022;
Rev-recd 25 01 2021;
Rev-recd 01 02 2022;
Rev-recd 30 06 2022;
Accepted 15 07 2022
ABSTRACT
Background:
Due to the increasing prevalence of kidney transplantation, a greater awareness of
variations in the surrounding vasculature is of surgical importance. During embryological
development, both the renal and gonadal arteries arise from the lateral mesonephric
branches of the dorsal aorta. In adults, gonadal arteries are paired vessels that
normally arise from the aorta at the level of the second lumbar vertebra.
Methods:
Routine cadaveric dissection completed by first-year medical and dental students incidentally
revealed anatomical anomalies.
Results:
We describe two cadaveric findings in males that demonstrate unilateral and bilateral
variations of testicular arteries originating from an aberrant renal artery in one
case and an accessory renal artery in the second case.
Conclusion:
By increasing awareness of anomalous testicular arteries, we aim to encourage the
standardization of preoperative vasculature exploration to minimize intraoperative
risk to living male kidney donors and increase patients’ understanding of the potential
risks and complications prior to consenting to the procedure, providing more accurate
information prior to surgery.
Keywords:
Renal Transplantation;
Congenital Abnormality;
Medical Imaging;
Dissection (Source: MeSH-NLM).
Highlights:
- These cases highlight the clinical relevance of post-mortem finding of anomalous origins
of the testicular artery in conjunction with aberrant renal vasculature.
- Acknowledging the existence of the aforementioned vascular anomalies becomes clinically
relevant for male kidney donors, as ligation of renal vasculature during surgery poses
a post-surgical risk to the testes in such cases.
Introduction
The number of kidney transplants performed globally has increased each year. The number
of transplants were: 17,611 in 2015, 19,061 in 2016, 21,028 in 2017, 22,393 in 2018,
and 24,273 in 2019.1,2 Over the past decade, 62% of countries have reported at least a 50% increase in the
number of living kidney donor transplants.3 The outcomes of recipients of deceased donors include a one-year survival rate of
95.4%, five-year survival of 79.7%, and an adjusted ten-year survival rate of 49.2%.
In comparison, the outcome of recipients of living donors had a one-year survival
rate of 98.8%, five-year survival of 88.0%, and an adjusted ten-year survival rate
of 61.5%.2 Given the greater potential for survival with living donors, it is important to create
a standard screening and informed consent process to increase the safety of living
donors, which may lead to more individuals feeling safe enough to sign up as living
donors.2
In the embryo, three sets of lateral mesonephric arteries branch off the aorta: caudal,
middle, and cranial. As the kidney ascends from its initial position in the pelvis
to its more cranial position in the abdomen, its arterial supply also transitions
from caudal to middle to cranial. Typically, the last branch of the middle group or
the first branch of the caudal group becomes the main renal artery while the rest
regress.4 It is postulated that if one of the rest of the branches does not regress, they may
persist as accessory renal arteries.5 Of the caudal arteries, one will typically persist and differentiate into the gonadal
artery. It is speculated that if one of the middle groups of lateral mesonephric arteries
persists, it will give rise to a gonadal artery that originates from the main or accessory
renal artery rather than the aorta.6
There are numerous possible embryological manifestations of gonadal and renal artery
anatomy. There are many proposed classification systems for these manifestations,6–8 most comprehensively by Kayalvizhi et al., who proposed a system that indicates four
primary groups of variations: Group I arising from the abdominal aorta, Group II arising
from the renal trunk, Group III arising from a suprarenal branch, and Group IV arising
from any other vasculature. According to this system, further classification can be
made on the basis of specific branch points in Groups I and II.9
Renal arteries normally arise from the abdominal aorta at the level of the second
lumbar vertebra.10 In both sexes, the gonadal arteries arise from the anterolateral surface of the abdominal
aorta, typically below the level of origin of the renal artery but superior to the
origin of the inferior mesenteric artery (L3).10
In both sexes, the gonadal artery travels along the superficial surface of the psoas
major muscle in the retroperitoneum.10 In males, the gonadal artery is known as the testicular artery, which enters the
inguinal canal through the deep inguinal ring, where it proceeds down to the testes.10 In addition to supplying oxygenated blood to the testes, the testicular artery is
involved in countercurrent temperature exchange with the pampiniform plexus of veins.
This venous plexus functions to maintain a slightly cooler temperature within a very
narrow range to accommodate spermatogenesis. In females, the gonadal artery is referred
to as the ovarian artery. It travels superficially to the psoas major muscle, down
the suspensory ligament of the ovary, enters the mesovarium, and may form an anastomosis
with the uterine artery in the broad ligament.10
A common variation of renal vasculature is the presence of an “accessory renal artery,”
which has been estimated to be prevalent in up to 30% of the general population.11 The accessory renal artery follows the path of the main renal artery to the renal
hilum and arises from the aorta either below or above the main renal artery. A second
known variation is an “aberrant renal artery,” which differs from an accessory renal
artery in that it crosses anteriorly to the inferior vena cava instead of posteriorly.12
Variations in renal vasculature are often accompanied by variations in gonadal artery
origin. In this report, the following anomalies of gonadal artery vasculature and
how they relate to variations in renal vasculature will be outlined: gonadal arteries
arising from (1) a normal renal artery, (2) an aberrant renal artery, and (3) an accessory
renal artery.
Methods
During cadaveric dissection by first-year medical and dental students at the Department
of Anatomy and Cell Biology at New York Medical College, USA, 58 cadavers were dissected
during the 2018-2020 academic years. All cadavers were well-embalmed and provided
for academic dissection. Cadavers were chosen for inclusion based on availability
sampling. In 2018, 21 female and 8 male cadavers were dissected. In 2019, 11 male
and 18 female cadavers were dissected. Cadavers dissected prior to 2018 were excluded
due to insufficient data collection. Cadavers with unidentifiable gonadal arteries
due to improper dissection were also excluded. In routine academic dissection, the
retroperitoneal structures of each cadaver were dissected following the instructions
outlined in Grant's dissector.13
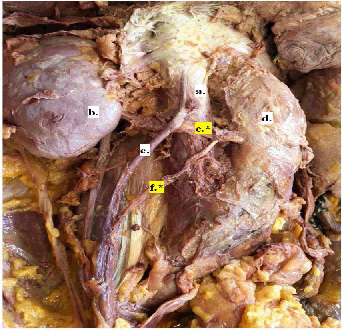
Figure 1.
2018 Male Cadaver; Left Gonadal Artery with Origin on Accessory Renal Artery, Right
Gonadal Artery with Origin on Normal Renal Artery.

Legend: a. Superior mesenteric artery; b. Left adrenal vein; c. Left renal artery; d. Left
kidney; e.* Left accessory renal artery; f. Left renal vein; g. Aorta; h. Left gonadal vein;
i.* Left gonadal artery; j. Ureter; k. Right kidney; l. Right renal vein; m. Left renal
vein; n. Right renal artery; o.* Right gonadal artery; p. Right gonadal vein; q. Inferior vena cava; r. Aorta.
The dissection occurred as follows: First, the posterior abdominal viscera were palpated,
and the parietal peritoneum was removed. The renal fascia and the kidneys were opened,
and the suprarenal glands were noted. Next, the testicular arteries were identified
at the deep inguinal ring. The arteries were dissected cleanly, progressing superiorly
along the retroperitoneal space to their origin on the abdominal aorta. Next, the
renal veins were identified and followed to the inferior vena cava. Finally, the renal
arteries were identified posteriorly to the renal veins and followed back to the abdominal
aorta. Upon completion of the cadaveric dissection, variants of the renal and testicular
arteries were noted and recorded.
Results
One male cadaver (2018) was incidentally identified to have bilateral variants with
a left accessory renal artery (figure 1e) from which the left testicular artery originated (figure 1i), and a single right renal artery from which the right testicular artery originated
(Figure 1o) A second male cadaver (2019) was also incidentally identified to have a unilateral
variant on the right side where the testicular artery originated (Figure 2f) from an aberrant renal artery (figure 2C). The renal vasculature of the second cadaver dissected in 2019 was not observed
on the left side due to prior dissection of the entire kidney and associated vasculature.
Figure 2.
2019 Male Cadaver; Right Gonadal Artery with Origin on Aberrant Renal Artery.
Legend: Inferior vena cava b. Right kidney c. * Aberrant right renal artery d. Aorta e. Right gonadal vein f.* Right gonadal artery
In summary, 2 out of the 19 males presented with variations of anomalies in renal
or testicular vasculature. No renal or ovarian vasculature anomalies were observed
in the 39 females in our cohort. Other demographic, clinical, and social characteristics
of the cadaver population were not known.
Discussion
Classification of Variations in Testicular and Renal Arteries
Using the Kayalvizhi et al. classification system,11 the variations observed in our cadaver study are classes IIB (from an accessory renal
branch) and IIC (from an aberrant renal branch).
Prevalence
In our cohort, two male cadavers presented with variations of anomalies in renal or
testicular vasculature, suggesting a prevalence of approximately 10.5% among males;
and no anomalies existed in the female subgroup, suggesting an overall prevalence
of 3.4%. This is considerably less than some of the reported estimates in the literature,12 presumably due to the small sample size. The higher prevalence in males corresponds
well with previous findings,7 which report a higher prevalence on the left side (81.23%) compared to the right
side in cases with unilateral anomalous arteries.14 One study found that 25% of cases occurred bilaterally.14
Method of Identification and Significance
Understanding atypical anatomic presentations of the renal and gonadal vasculature
is essential prior to renal and testicular surgery in order to mitigate the associated
risks. We speculate that the surgical risks posed by the unfamiliar vasculature during
nephrectomy, including longer operation times, increased blood loss, and greater risk
of complications associated with unfamiliar vasculature,15 are greater for male kidney donors because the testicular artery provides the most
significant blood flow to the testes. Aside from their surgical significance, the
variations in renal vasculature or origin of the gonadal vasculature do not present
with any clinical manifestations and are often discovered during surgery or post-mortem.
Imaging
There is currently no standardized imaging in the United States for the preoperative
screening of a kidney donor. However, magnetic resonance imaging (MRI) and computed
tomography scan (CT) are commonly used to assess the anatomy of the kidney, vasculature,
and urinary collecting system in living donors.16 Although necessary for the preoperative workup, both MRI and CT are considered suboptimal
for visualizing anomalous gonadal vasculature involving the kidney.17 CT Angiography remains the gold standard for visualizing the gonadal and renal vessels,
although it is not universally used.18 Doppler ultrasound of the renal hilum is a quick and effective diagnostic procedure
that could be alternatively implemented to screen for the presence of atypical renal
vasculature prior to surgery for male kidney donors after a statistically significant
proof of a higher risk to male donors has been established. This method is preferred
over conventional sonography as it provides functional and vascular information, locating
the presence and blood flow of vessels. It can also be a useful tool to assess the
donor organ for fibrosis, masses, and chronic kidney disease.19 Research suggests that the presence of a gonadal artery with anomalous origin is
often associated with variants in renal vasculature.15,20 The presence of atypical renal vasculature in the living donor might raise suspicion
in the physician regarding variants in gonadal vasculature, and therefore, arteriography
can be performed to further investigate the anatomy of the gonadal arteries. As the
global rate of kidney transplantation has risen in past decades,2 a standardization of screening methods to minimize risks for transplant candidates
is increasingly important.
In addition to the lack of preoperative imaging, there is currently no standardized
procedure for obtaining informed consent from the donor for nephrectomy, and research
has shown that donors often make their decision based on moral beliefs and without
full knowledge of the scope of potential complications.21
Surgical Risks
Variations in renal and gonadal vasculature are associated with intraoperative and
postoperative risks to living donors. In living donors, if a variation in gonadal
vasculature is unknown prior to surgery, it may result in the ligation of the donor's
gonadal artery at the surgeon's discretion in the process of obtaining the donor kidney.
In a male, the testicular artery provides the most significant blood flow to the corresponding.
We speculate that ligation of this artery could contribute to the possible risk of
complications to the testes following the procedure, including loss of the temperature
regulation system of the testes. The ovaries have a dual blood supply supported by
the ovarian and uterine arteries, lowering their potential risk of cutting complete
supply to the female gonads. Post-operative outcomes of testicular ligation are not
well-reported, so the risks of ligation remain unclear. However, current available
research suggests that the incidence of adverse outcomes to the testes following testicular
artery ligation is low.13,14,21,22 Vascular variations such as these are of significance with the implementation of
laparoscopic procedures, as unfamiliar anatomy in the surgical field is a common contributing
factor to intraoperative complications.23 In laparoscopic donor nephrectomy, multiple arteries were associated with longer
operation times and increased blood loss during surgery.20 In surgeries other than donor nephrectomy, certain variants of anomalous gonadal
vasculature are absolute contraindications for surgical treatment; for example, a
gonadal artery originating from the inferior polar renal artery can be a major contraindication
for percutaneous treatment of the syndrome of the pyelo-ureteral junction.14 Without preoperative awareness of the existence of certain variants in the vasculature
and the risks associated with them, the donor is left to consent without full knowledge
of the scope of potential intraoperative and postoperative complications.
Previously, to reduce the risk of unintentional ligations, surgeons would perform
a “time-out” where all key structures would be identified. While implementing this
strategy decreases the incidence of gonadal artery ligation, adding a pre-procedure
US-Doppler could cut down on operation time, hence enhancing overall institutional
efficiency, and over time have a beneficial impact financially due to reduced time
per procedure in the operating room.
Limitations
Our estimates for the prevalence of atypical gonadal and renal vasculature are limited
by availability sampling and the incidental nature of their discovery. In this observational
study, all provided cadavers were screened according to our inclusion and exclusion
criteria, as previously mentioned. Our cases were discovered by medical students who
are in the process of familiarizing themselves with the procedures of cadaveric dissection
and with typical anatomy. This is a potential source of selection bias, as candidates
for inclusion may have been damaged in the process. Nonetheless, no cadavers met exclusion
criteria and all were dissected under the direct supervision of physicians. The prevalence
results of this sample cannot be generalized to the general population due to the
small size and potential of misrepresentation in the sample compared to the general
population. We accounted for the limited number of cadavers studied at the school
by including data from multiple years to increase our sample size. Furthermore, due
to limited access to cadaver demographic information, no conclusions can be made on
how these results can be applied to specific demographic populations. Given the frequent
clinical insignificance of these anomalies for the general population, much of our
knowledge of these variants and their respective prevalence comes from postmortem
findings. We acknowledge that our results have limited external validity because of
the aforementioned limitations, but are clinically valuable to raise awareness of
the necessity of renal and gonadal vasculature screening.
Summary – Accelerating Translation
Title: A Cadaveric Study on the Anomalous Origin of Renal and Gonadal Vasculature: An Observational
Study.
Main problem to solve: Due to the increasing prevalence of kidney transplantation, a greater awareness of
variations in the surrounding vessels is important to surgeons and patients. During
development, the renal and gonadal arteries arise from branches of the same vessels.
In adults, gonadal arteries are paired vessels that normally arise from the aorta.
Variations in vessels can be detected prior to surgery with imaging, although currently,
there is no agreed-upon standard of pre-surgical screening.
Aim of study: To illustrate variations in vessels that supply the kidneys and the gonads; to explore
the benefits of different imaging modalities.
Methodology: Routine cadaveric dissection completed by first-year medical students and dental
students incidentally revealed anatomical variations.
Results: We describe two cadaveric findings in male cases which demonstrate unilateral and
bilateral variations of testicular arteries originating from an aberrant renal artery
in one case and an accessory renal artery in the other.
Conclusions: By increasing awareness of variations in testicular arteries, we hope to encourage
the standardization of preoperative vasculature exploration to minimize intra-operative
risk to living male kidney donors and increase patients’ understanding of potential
risks and complications prior to consenting to the procedure, providing more accurate
information prior to surgery.
Acknowledgments
Roger B. Bender MPH provided cadaver demographic data for the Department of Anatomy
and Cell biology at New York Medical College for the years 2018-2019.
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Compliance with ethical standards
This study has authorization for the use of cadaveric images for educational publications.
This study does not contain any identifiable patient data which would require informed
consent. This study was in accordance with the Human Anatomy Act of the laws of the
United States of America.
Author Contributions
Conceptualization: DQ; Supervision: DP; Writing – Original Draft Preparation: PF,
DM, LB, JN, DQ; Writing – Review & Editing: PF, DM.
References
1. Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, et. al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am J Transplant. 2021;21 Suppl 2:21–137.
2. United States Renal Data System [Internet]. 2020 [cited 2021 Sep 26]. USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney
Diseases, Bethesda, MD, 2020. Available from: https://adr.usrds.org/2020/end-stage-renal-disease/6-transplantation.
3. Horvat LD, Shariff SZ, Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network. Global trends in the rates of living kidney donation. Kidney Int. 2009;75(10):1088–98
4. Perez, JA, Torres FG, Toribio AM, Fernandez LK, Hayoun, C, Naranjo ID. Angio CT assessment of anatomical variants in renal vasculature: its importance in
the living donor. Insights Imaging. 2013; 4:199–211.
5. Sadler, TW. Langman's medical embryology. 14th ed. Philadelphia: Lippincot Williams & Wilkins, 2015.
6. Cicekcibasi AE, Salbacak A, Seker M, Ziylan T, Buyukmumcu M, Uysal II. The origin of gonadal arteries in human fetuses: Anatomical variations. Ann Anat. 2002;184:275–9.
7. Machnicki A, Grzybiak M. Variations in testicular arteries in fetuses and adults. Folia Morphol (Warsz). 1997;56(4):277–85.
8. Notkovich H. Testicular artery arching over renal vein: clinical and pathological considerations
with special reference to varicocele. Br J Urol. 1955;27(3):267–71.
9. Kayalvizhi I, Narayan RK, Kumar P. Anatomical variations of testicular artery: a review. Folia Morphol (Warsz). 2017;76(4):541–50.
10. Moore KL, Dalley AF. Clinically oriented anatomy. Philadelphia: Lippincott Williams & Wilkins. 1999.
11. Praveen, Kumar G (2012) Bilateral Superior Accessory Renal Arteries - Its Embryological Basis and Surgical
Importance – A Case Report. J Clinic Case Reports 2:e105.
12. Graves, F.T. The aberrant renal artery. J Anat 1956; 90(Pt 4): 553–8.1.
13. Tank, PW. Grant's dissector. 15th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
14. Petru B, Elena S, Dan I, Constantin D. The morphology and the surgical importance of the gonadal arteries originating from
the renal artery. Surg Radiol Anat. 2007;29(5):367–71.
15. Das, S. Anomalous renal arteries and its clinical implications. Bratisl Lek Listy 2008;109(4):182–4.
16. Harmath CB, Wood CG 3rd, Berggruen SM, Tantisattamo E. Renal Pretransplantation Work-up, Donor, Recipient, Surgical Techniques. Radiologic clinics of North America. 2016;54(2):217–34.
17. Kok NF, Dols LF, Hunink MG, et al. Complex vascular anatomy in live kidney donation: imaging and consequences for clinical
outcome. Transplantation. 2008;85(12):1760–5.
18. Aghayev A, Gupta S, Dabiri BE, Steigner ML. Vascular imaging in renal donors. Cardiovasc Diagn Ther. 2019;9(Suppl 1):S116–S130.
19. Gameraddin, M. Ultrasound of the Kidneys: Application of Doppler and Elastography, Essentials of
Abdominal Ultrasound. Books on Demand; 2019.
20. Gesase, A.P. Rare origin of supernumerary renal vessels supplying the lower pole of the left kidney. Ann Anat. 2007;189(1):53–8.
21. Kortram K, Lafranca JA, IJzermans JN. Dor FJMF. The need for a standardized informed consent procedure in live donor nephrectomy:
a systematic review. Transplantation 2014; 98: 1134–43.
22. Chan P, Wright E, Goldstein M. Incidence and postoperative outcomes of accidental ligation of the testicular artery
during microsurgical varicocelectomy. J Urol. 2005;173(2):482–4.
23. Hsu CW, et al. Incidence and clinical outcomes of gonadal artery injury during colorectal surgery
in male patients. J Gastrointest Surg. 2019; 23: 2075–80.
24. Gupta R, Gupta A, Aggarwal N. Variations of gonadal veins: embryological prospective and clinical significance. J Clin Diagn Res. 2015;9:AC08–10.
Penelope C. Farris, 1 B.S. Fourth-year Medical Student. New York Medical College, Valhalla, United States
of America.
Dylan M. Macciola, 2 B.S. Fourth-year Medical Student. New York Medical College, Valhalla, United States
of America.
Lauren N. Barazani, 3 M.S. Fourth-year Medical Student. New York Medical College, Valhalla, United States
of America.
Justin R. Nathan, 4 B.A. Fourth-year Medical Student. New York Medical College, Valhalla, United States
of America.
Danielle Quinn, 5 M.D. Emory University, Atlanta, United States of America.
Daniel F. Peters, 6 M.D. New York Medical College, Valhalla, United States of America.
About the Author: Authors 1-4 are current medical students at New York Medical College in Valhalla,
New York, Author 5 is a resident at Emory University Hospital, and Author 6 is the
Foundational Science Course Director for Anatomy and Embryology at New York Medical
College.
Correspondence: Dylan M. Macciola. Address: New York Medical College, Valhalla, United States of
America. Email: Dylan.macciola@gmail.com
Editor: Vincent Kipkorir
Student Editors: Purva Shah, Lourdes Adriana Medina-Gaona & Vinso Chan
Copyeditor: Joseph Tonge
Proofreader: Sebastian Diebel
Layout Editor: Anna-Maria Chantaliyska
Process: Peer-reviewed
Cite as: Farris PC, Macciola DM, Barazani LN, Nathan JR, Quinn D, Peters DF. A Cadaveric Study
on the Anomalous Origin of Renal and Gonadal Vasculature: An Observational Study.
Int J Med Stud. 2022 Jul-Sep; 10(3):316-20.
Copyright © 2022 Penelope C. Farris, Dylan M. Macciola, Lauren N, Barazani, Justin
R. Nathan, Danielle Quinn, Daniel F. Peters
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 3, September 2022

