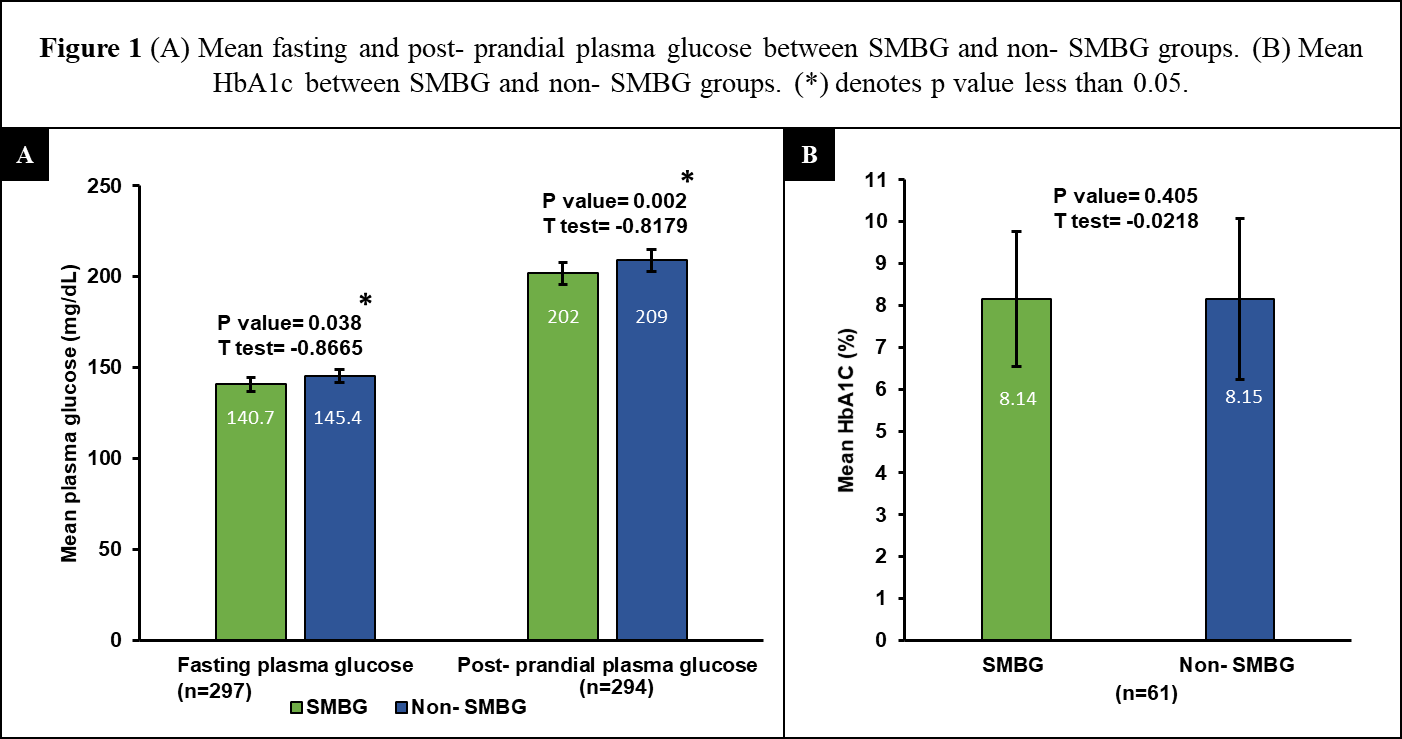
Association between glycemic levels and SMBG use. (A) Mean fasting and Post-prandial plasma glucose between SMBG and Non-SMBG groups. (B) Mean HbA1c between SMBG and Non-SMBG groups. (*) denotes significant difference (p value less than 0.05).
Andrew Thomas1, Mohan T Shenoy2, K.T. Shenoy3, Nirmal George4
doi: http://dx.doi.org/10.5195/ijms.2021.786
Volume 9, Number 2: 140-144
Received 08 10 2020: Rev-request 31 10 2020: Rev-request 12 12 2020: Rev-request 06 02 2021: Rev-recd 17 11 2020: Rev-recd 12 12 2020: Rev-recd 17 02 2021: Accepted 06 04 2021
ABSTRACT
Background:The effectiveness of self-monitoring of blood glucose (SMBG) in type 2 diabetes mellitus (T2DM) patients is debated in the literature. We aimed at elucidating the association and patterns of complications between SMBG use and plasma glucose values.
Methods:This cross-sectional study comprised 303 participants from outpatient departments with T2DM for over 12 months. We analyzed sociodemographic and clinical variables including: anthropometry, SMBG use, disease duration, treatment modality, complications, plasma glucose level, and glycated hemoglobin level (%).
Results:The mean duration of T2DM was 93 ± 76 months. Participants were grouped into SMBG users (n=115, 38%) and non-SMBG users (n=188, 62%). The mean fasting plasma glucose levels of SMBG and non-SMBG users were 140.7±42.7 (95% Confidence Interval [95%CI]: 132.72;148.67) mg/dl and 145.4±50 (95%CI: 138.12;152.67) mg/dl (p=0.03), respectively. The mean post-prandial plasma glucose levels of the SMBG and non-SMBG groups were 202 ± 63.42 (95%CI: 190.23;213.76) mg/dl and 209±84.54 (95%CI: 196.56;221.43) mg/dl (p=0.002), respectively. The mean difference in HbA1c among the groups were 8.14±1.69% (95%CI: 7.59;8.68) and 8.15±1.98% (95%CI: 7.27;9.02) (p=0.4), respectively. Hypoglycemia (n=50, 43.5%) was the most common complication. The prevalence of neuropathy (n=5, 4.3%, p=0.036) and cardiovascular disease (n=21, 18.3%, p=0.042) were significantly higher in the SMBG group.
Conclusion:Although plasma glucose values were significantly lower in the SMBG group, its clinical significance remains questionable. Furthermore, many participants in both groups had shortfalls in awareness, monitoring, and glycemic control. SMBG use needs to be evaluated in a cohort of patients with T2DM with adequate health awareness.
Keywords: Blood Glucose Self-Monitoring; Type 2 Diabetes Mellitus; Glycemic Control; Blood glucose; Diabetes Complications (Source: MeSH-NLM).
India is a country with a high incidence of diabetes mellitus. The morbidity and mortality related to this disease are enormous and pose a significant burden on the public health of this country in the future.1 Venous plasma and capillary whole blood methods are two ways of estimating blood glucose level. The venous plasma glucose level is slightly higher in random and fasting glucose estimation, but lower than capillary whole blood glucose level for samples taken 2 hours after glucose is given orally. However, the diagnostic criteria are similar between these two methods of estimation.2 According to the American Diabetic Association (ADA), random blood glucose values of 79–140 mg/dl are considered normal; 140–200 mg/dl, pre-diabetes; and a value above 200 mg/dl, diabetes. In terms of glycated hemoglobin levels, values less than 6.5% are normal, whereas values between 5.7% and 6.7% are considered high risk. 3, 4 Control of blood glucose is important in preventing diabetes-associated complications; however, over 70% of all patients diagnosed with diabetes have uncontrolled diabetes.5
Some may argue that self-monitoring of blood glucose (SMBG) can be recommended for all patients with type 2 diabetes mellitus (T2DM).6 This is because SMBG can be useful in detecting hypoglycemia and hyperglycemia. Furthermore, high blood glucose levels encourage patients to improve their diet and physical activity. Multiple SMBG measurements taken over a period of time may also be helpful for physicians to monitor their patients and modify their treatment if needed.6, 7 Glycemic variability is a term used to describe the fluctuations of glucose levels. While SMBG may provide diurnal glucose profile, continuous glucose monitoring (CCM) is considered helpful in detecting glycemic variability.8
The data on SMBG practice among patients with T2DM are not well understood. Many studies have been conducted to estimate the effectiveness of SMBG practice, especially among non-insulin-treated patients. Some studies revealed no association between in SMBG and glycemic control, whereas others indicated the benefits of SMBG use.9–14 A reduction in hemoglobin A1C (HbA1C) of 0.5% was found when the patients were better educated to interpret the values of SMBG.7 Physicians need to monitor patients with T2DM regularly, because the effectiveness of SMBG is dependent both on patients and appropriate glucometer use.15 Patients should be made aware that practicing SMBG alone will not improve their glycemic control. Good glycemic control is attained only when data obtained are properly interpreted to modify treatment strategies. This study aimed at elucidating the use of SMBG in patients with T2DM by comparing glycemic levels and complication rates among SMBG and non-SMBG users.
This was a cross-sectional study conducted from May to June 2019. The project was approved by the Institutional Ethical Committee of Sree Gokulam Medical College and Research Foundation (SGMC-IEC no.: 34/450/05/2019). Each participant provided a written informed consent before the commencement of the study.
Sree Gokulam Medical College and Research Foundation (SGMC&RF) is a tertiary health center located at Thiruvananthapuram in the state of Kerala, India. Study participants were selected by convenient sampling from the outpatient departments of General medicine, General Surgery, Orthopedics, Endocrinology, Diabetology, and Gastroenterology of SGMC&RF. Participants with T2DM for over 12 months and those aged at least 18 years were included in the study. Pregnant women were excluded from the study.
Socio-demographics, anthropometry (height in cm and weight in Kg), duration of disease (in months), current treatment pattern [6 treatment modalities, namely insulin preparations, oral hypoglycemic drugs except metformin monotherapy, metformin monotherapy, alternative drug therapy like Ayurveda or Homeopathy, combination therapy (insulin and oral hypoglycemic drugs), and no treatment], recent plasma glucose values (fasting, post-prandial in mg/dl, and HbA1C as a percentage), co-morbidities, such as hypertension, dyslipidemia, hypo- and hyperthyroidism, bronchial asthma, cardiovascular disease (cardiac failure, arrhythmias, and myocardial infarction), stroke (both ischemic and hemorrhagic), neuropathy, nephropathy, and retinopathy, and pattern of SMBG use (duration, frequency) were the variables studied.
Awareness and practice of SMBG among the study participants were enquired and noted. Following data collection, all participants were educated on disease progression and appropriate SMBG practice.
Data were collected through interviews lasting 15 minutes conducted by the principal investigator, using questionnaires on SMBG practice. Height and weight were measured for calculating BMI. Laboratory records, such as blood glucose, lipid profile, and HbA1C, were perused from the electronic records database with patients' permission. Only their most recent blood values were used in the study for analysis. All eligible and consenting participants were recruited to minimize the selection and sampling bias.
The sample size was estimated based on a previous report15 using the Open-Epi (Open-Source Epidemiologic Statistics for Public Health)16 software. A proportion of 52.4% was assumed to be SMBG users with a precision of 5%, alpha error (0.05), and power of 90%; the required sample size was calculated. All quantitative variables were compared using the mean and standard deviation.
The association between SMBG practice and plasma glucose values was assessed using the independent t-test. Chi-square test and odds ratio with 95% Confidence Interval (95%CI) were used to determine the association between complications and SMBG practice. A sub-group analysis among the non-insulin-taking participants was performed to determine the association between SMBG use and glycemic values of non-insulin-taking participants. A p-value <0.05 was considered statistically significant. SPSS version 25 (Statistical Package for the Social Sciences, SPSS Inc., U.S.A) was used for all data analyses. Sensitivity analysis was not performed.
Overall, 303 participants were interviewed in our study, of which 171 (56.4%) were males and 132 (43.6%) were females. Age-wise, 141 (46.5%) and 162 (53.5%) participants were above and below 60 years, respectively (p=0.191). The total population age average was 59.61 ± 10.26 years. The mean duration of T2DM in our study sample was 93.5 months. The BMI of 294 participants were assessed using the Asian BMI criteria17 (Table 1). Nine participants were excluded from this analysis due to missing information, after which 147 (50%), 64 (21.8%), 76 (25.8%), and 7 (2.5%) participants were determined to be obese, overweight, normal (18.5 to 23 kg/m2), and underweight, respectively. No statistically significant difference in BMI was observed between SMBG and non-SMBG users (p=0.2).
Table 1.Participants Classified According Asian BMI Criteria
| Asian BMI criteria | SMBG group | Non-SMBG group |
|---|---|---|
| Underweight (<18.5 kg/m2) | 7 | 0 |
| Normal (18.5–23 kg/m2) | 44 | 32 |
| Overweight (23.1–27.5 kg/m2) | 37 | 27 |
| Obese (≥27.5 kg/m2) | 92 | 55 |
The study participants were classified into SMBG (115, 38%) and non-SMBG groups (188, 62%). Among the SMBG group, 96 (83.5%), 8 (7%), 5 (4.3%), 4 (3.5%), and 2 (1.7%) participants practiced SMBG irregularly, once weekly, once monthly, once daily, and multiple times a day, respectively.
A summary of different treatment modalities administered to the study participants is given in Table 2. Metformin monotherapy administered to 113 (37.3%) participants was the most common treatment modality, whereas 51 (16.8%) and 63 (20.8%) participants were on insulin monotherapy and combined therapy with insulin and oral hypoglycemic drugs, respectively. Participants were grouped based on insulin use for sub-group analysis. No significant difference was observed between them.
Table 2.Treatment Modalities Used by Study Participants for Control of Type 2 Diabetes Mellitus.
| Treatment | SMBG group (n=115) | Non-SMBG group (n=188) | p-value |
|---|---|---|---|
| Metformin | 30 (26.1%) | 83 (44.1%) | 0.002† |
| Monotherapy with oral anti-diabetic drugs (except metformin) | 13 (11.3%) | 20 (10.6%) | 0.86† |
| Combination of more than one oral anti-diabetic drugs | 7 (6.1%) | 11 (5.9%) | 0.93† |
| Insulin Preparations | 23 (20%) | 28 (14.9%) | 0.25† |
| Insulin and oral anti-diabetic drugs | 32 (27.8%) | 31 (16.5%) | 0.02† |
| Alternative medicine* | 3 (2.6%) | 1 (0.5%) | 0.15‡ |
| No medications | 7 (6.1%) | 14 (7.4%) | 0.65† |
Most of the participants (79, 68.7%) performed SMBG after overnight fasting, whereas 3 (2.6%) and 33 (28.7%) participants performed it 2 hours after eating and randomly, respectively. More than half (74, 64.3%) of the participants felt they were not adequately educated about SMBG use. Among the SMBG users, only 38 (33%) participants modified their treatment using SMBG values. The other 78 (67.8%) participants began exercising and had diet modifications because of SMBG use.
In all, 60 (52.2%) participants said they would verify the SMBG values by performing follow-up tests in certified laboratories. However, 48 (41.7%) participants did not confirm their SMBG values at any laboratories. Only 7 (6.1%) participants consulted physicians on their SMBG values.
The mean plasma glucose and HbA1c levels of the SMBG and non-SMBG groups were compared using t-test (Figure 1). The fasting plasma glucose levels of 297 participants were analyzed. Among them, 113 participants practiced SMBG. The mean plasma fasting glucose levels were 140.47±42.7 (95%CI: 132.72–148.67) mg/dl and 145.4±50 (95%CI: 138.12; 152.67).
Figure 1Association between glycemic levels and SMBG use. (A) Mean fasting and Post-prandial plasma glucose between SMBG and Non-SMBG groups. (B) Mean HbA1c between SMBG and Non-SMBG groups. (*) denotes significant difference (p value less than 0.05).

prandial (7 mg/dl, p=0.002) plasma glucose. HbA1c levels of the SMBG and non-SMBG groups were 8.14%±1.69 (95%CI: 7.59-8.68) and 8.15%±1.98 (95%CI: 7.27-9.02), respectively. However, no significant difference was observed in the mean HbA1c levels between both groups. A sub-group analysis with t-test was conducted for the non-insulin users. No statistically significant difference was identified between both groups, in terms of their fasting (p=0.4, t=-0.8), post-prandial (p=0.9, t=0.1), or HbA1c levels (p=0.7, t=0.3).
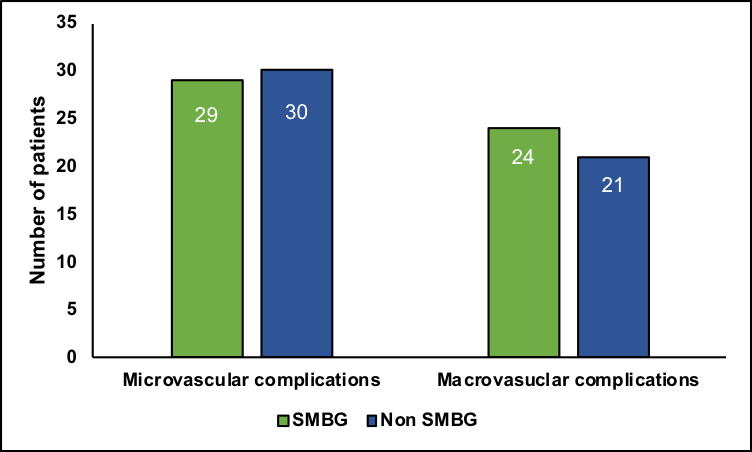
Table 3 summarizes the pattern of complications and co-morbidities. Out of the 303 participants, 117 (38%) had hypoglycemia, which was the most common complication (p=0.17). Fifty-nine (19.5%) participants had at least one micro-vascular (retinopathy, nephropathy, and neuropathy) complication, and 45 (14.8%) participants had at least one macro-vascular (cardiovascular diseases and stroke) complication.
Table 3.Frequency of complications and co-morbidities observed in study participants
| Complications and Co-morbidities | Total (n=303) | SMBG group (n=115) | Non-SMBG group (n=188) | P value |
|---|---|---|---|---|
| Retinopathy | 54 (17.8%) | 25 (21.7%) | 29 (15.4%) | 0.16 |
| Nephropathy | 10 (3.3%) | 5 (4.3%) | 5 (2.7%) | 0.42 |
| Neuropathy | 20 (6.6%) | 12 (10.4%) | 8 (4.3%) | 0.04 |
| Hypoglycemia | 117 (38.6%) | 50 (43.5%) | 67 (35.6%) | 0.17 |
| Cardiovascular disease | 40 (13.2%) | 21 (18.3%) | 19 (10.1%) | 0.04 |
| Stroke | 8 (2.6%) | 4 (3.5%) | 4 (2.1%) | 0.48 |
| Hypertension | 151 (49.8%) | 49 (42.6%) | 102 (54.3%) | 0.04 |
| Dyslipidemia | 141 (46.5%) | 51 (44.3%) | 90 (47.9%) | 0.55 |
| Thyroid dysfunction | 45 (14.9%) | 15 (13%) | 30 (16%) | 0.49 |
More participants who practiced SMBG had neuropathy (n=12, 10.4%, p=0.03) and cardiovascular disease (n=21, 18.3%, 0.04). The odds ratios (OR) of micro-vascular (OR=1.8, 95%CI=1.00–3.2) and macro-vascular complications (OR=2.1, 95%CI=1.1–3.9) were significantly different when analyzing SMBG and non-SMBG groups, as summarized in Figure 2. Micro-vascular and macro-vascular complications were prevalent in both the non-SMBG and SMBG groups.
Figure 2Frequency of micro-vascular and macro-vascular complications among SMBG and non-SMBG users. (a) denotes odds ratio 1.8, 95%Cl 1.001;3.2, p=0.048; (b) denotes Odds ratio 2.1, 95%Cl 1.1;3.9, p=0.021.
The usefulness of SMBG practice among patients with T2DM, especially those without insulin medication, is doubtful in the literature. We conducted a cross-sectional study among 303 patients with T2DM patients with and without SMBG practice, and compared their fasting, post-prandial and HbA1c values. The mean HbA1c values did not differ between the two groups. While the mean plasma glucose values between the groups may be statistically significant, the differences were too infinitesimal to produce a clinically significant difference. A mere 7 mg/dl difference in post-prandial glucose is doubtful to elicit a clinically significant difference in practice.18
One of the specific goals for practicing SMBG is to detect and prevent hypoglycemia.20 Contrary to this, hypoglycemia was the most common complication observed in the participants. Furthermore, no statistically significant difference existed in the proportion of hypoglycemia between SMBG and non-SMBG groups, as reported in literature.21
Most of the complications, except neuropathy and cardiovascular disease, had no statistically significant differences between the two groups. The leading cause of mortality and morbidity in patients with diabetes is related to cardiovascular events.22 A weak, yet significant association between SMBG use and cardiovascular disease has been reported.23 Huang et al. 24 observed that frequent SMBG monitoring decreased micro-vascular complications. One interesting finding was that neuropathy and cardiovascular diseases were more prevalent in the SMBG group with statistical significance. Overall, our study observed that more micro-vascular complications were present in the non-SMBG group (29 vs. 30), whereas more macro-vascular complications were present in the SMBG group (24 vs. 21). However, these complications may not be associated with SMBG use. A possible explanation for this finding might be that participants with more complications used SMBG because of severe disease.
SMBG use in T2DM is debated in the literature. Improvement in glycemic control can only be found if the patient is educated about the appropriate ways of practicing SMBG.25, 26 Many patients in our study were not satisfied with the health education they received from the healthcare provider. Most patients practiced SMBG irregularly and did not confirm or consult physicians on results. This might be the reason for poor response to SMBG practice in our study setting. Perhaps, the most concerning finding in our study was that mean fasting, post-prandial, and HbA1C of both groups were significantly higher than the recommended glycemic targets by American Diabetes Association (ADA), American College of Endocrinologists, and International Diabetes Federation (IDF).19 In India, education on diabetes is not enough to impart adequate awareness and monitoring status of glycemic control.27 Thus, determining the efficiency of SMBG is challenging.
The effectiveness of SMBG especially among patients with T2DM who are not on insulin therapy is debated in the literature. A systematic review and meta-analysis conducted in 2012 included 12 randomized controlled trials. They concluded that the effect of SMBG lasted only about six months in these patients. Furthermore, they also observed that the effectiveness decreases after one year.28 Another multicenter analysis on 24,500 patients from 191 centers observed that patients with T2DM on oral hypoglycemic agents or nutrition therapy derived no apparent benefits from SMBG.29 These findings are similar to those of our study. One prospective study conducted among 689 patients observed benefits in patients who performed SMBG, but observed a 0.3% reduction in HbA1c level (8.1 ± 1.6% vs. 8.4 ± 1.4%, p = 0.012). Thus, any claims of significant clinical effects of SMBG among SMBG users are doubtful.13
The study had few limitations. First, information bias due to reliance on self-declared information was most likely. Second, ideally, a randomized controlled trial would be better to ascertain the differences between the SMBG and non-SMBG groups. Third, while we only included participants who practiced SMBG for at least 6 months, we did not analyze the duration of SMBG use with their glycemic values.
Even though our study indicated a significant difference in fasting and post-prandial plasma glucose, participants had shortfalls in awareness, practice, and control of diabetes. Further studies using randomized controlled trials and meta-analysis should be conducted on patients with T2DM using SMBG to determine its effectiveness and establish standard guidelines for SMBG practice.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization, Resources & Supervision: AT, MTS, KTS. Data Curation: AT. Investigation: AT, MTS. Project Administration & Validation: AT, KTS. Formal Analysis, Methodology, Writing – Original Draft Preparation & Writing – Review & Editing: AT, MTS, KTS, NG.
We acknowledge Mr. Jayakumar, for help with statistical analysis. This study was presented in NEOPHARMACON 2019, at Sree Gokulam Medical College and Research Foundation, Kerala, India
1. Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014 Jan 31;7(1):45–8.
2. Colagiuri S, Sandbaek A, Carstensen B, Christensen J, Glumer C, Lauritzen T, et al. Comparability of venous and capillary glucose measurements in blood. Diabet Med. 2003 Nov;20(11):953–6.
3. Nair M, Prabhakaran D, Narayan KM, Sinha R, Lakshmy R, Devasenapathy N, et al. HbA(1c) values for defining diabetes and impaired fasting glucose in Asian Indians. Prim Care Diabetes. 2011 Jul;5(2):95–102.
4. Thomas T, Prabhata S, Valsangkar S. Diabetes screening and the distribution of blood glucose levels in rural areas of North India. J Family Community Med. 2015 Sep–Dec;22(3):140–4.
5. Mohan V, Mapari J, Karnad P, Mann J, Maheshwari V. Reduced diabetes mellitus-related comorbidities by regular self-monitoring of blood glucose: Economic and quality of life implications. Indian J Endocr Metab. 2018;22(4):461.
6. Malanda UL, Bot SD, Nijpels G. Self-Monitoring of Blood Glucose in Noninsulin-Using Type 2 Diabetic Patients: It is time to face the evidence. Diabetes Care. 2013 Jan 1;36(1):176–8.
7. AADE7™ Self-Care Behaviors. American Association of Diabetes Educators (AADE) Position Statement. 2014. Available from: https://www.diabeteseducator.org/docs/default-source/legacy-docs/_resources/pdf/publications/aade7_position_statement_final.pdf?sfvrsn=4. Last updated December 03, 2014; cited Jun 29, 2019.
8. Ceriello A, Monnier L, Owens D. Glycaemic variability in diabetes: clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019 Mar;7(3):221–230.
9. Young LA, Buse JB, Weaver MA, Vu MB, Mitchell CM, Blakeney T et al. Glucose Self-Monitoring in Non-Insulin-Treated Patients With Type 2 Diabetes in Primary Care Settings: A Randomized Trial. JAMA Intern Med. 2017 Jan;177(7):920–29.
10. Wang J, Zgibor J, Matthews JT, Charron-Prochownik D, Sereika SM, Siminerio L. Self-Monitoring of Blood Glucose Is Associated With Problem-Solving Skills in Hyperglycemia and Hypoglycemia. Diabetes Educ. 2021 Mar 1;38(2):207–18.
11. Farmer A, Balman E, Gadsby R, Moffatt J, Cradock C, McEwen L, et al. Frequency of Self-Monitoring of Blood Glucose in Patients with Type 2 Diabetes: Association with Hypoglycaemic Events. Curr Med Res Opin. 2008 Nov 1;24(11): 3097–104.
12. Iwuala SO, Olamoyegun MA, Sabir AA, Fasanmade OA. The relationship between self-monitoring of blood glucose and glycaemic control among patients attending an urban diabetes clinic in Nigeria. Ann Afr Med. 2015 Oct–Dec;14(4):182–7.
13. Guerci B, Drouin P, Grangé V, Bougnères P, Fontaine P, Kerlan V, et al. Self-Monitoring of Blood Glucose Significantly Improves Metabolic Control in Patients with Type 2 Diabetes Mellitus: The Auto-Surveillance Intervention Active (ASIA) Study. Diabetes Metab. 2003 Dec;29(6):587–94.
14. American Diabetes Association. Standards of medical care in diabetes. 2019. Available from https://care.diabetesjournals.org/content/diacare/suppl/2018/12/17/42.Supplement_1.DC1/DC_42_S1_2019_UPDATED.pdf. Last updated January, 2019; cited Jun 29, 2019.
15. Mast O, Tan A, Punjabi K. Usage of Self-Monitoring of Blood Glucose (Smbg) By Diabetes Therapy Type in India. Value Health. 2014 Nov;17(7):A362.
16. Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. www.OpenEpi.com, cited Apr 24, 2019.
17. Heslehurst N, Sattar N, Rajasingam D, Wilkinson J, Summerbell CD, Rankin J. Existing maternal obesity guidelines may increase inequalities between ethnic groups: a national epidemiological study of 502,474 births in England. BMC Pregnancy Childbirth. 2012 Dec 18;12:156.
18. Ranganathan P, Pramesh CS, Buyse M. Common pitfalls in statistical analysis: Clinical versus statistical significance. Perspect Clin Res. 2015 Jul–Sep;6(3):169–70.
19. Monnier L, Colette C. Target for glycemic control: concentrating on glucose. Diabetes Care. 2009 Nov;32 Suppl 2(Suppl 2):S199–204.
20. Czupryniak L, Barkai L, Bolgarska S, Bronisz A, Broz J, Cypryk K, et al. Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in Central and Eastern Europe–recommendations from the international Central-Eastern European expert group. Diabetes Technol Ther. 2014 Jul;16(7):460–75.
21. O'Kane MJ, Bunting B, Copeland M, Coates VE, ESMON study group. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008 May 24;336(7654):1174–7.
22. International Diabetes Federation. Fact Sheet: Diabetes and Cardiovascular Disease (CVD). Available from https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html. Last updated June 29, 2019; cited Jun 29, 2019.
23. Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, et al. ADAG Study Group. HbA1(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2011 Jan;54(1):69–72.
24. Huang IC, Wang PW, Liu RT, Tung SC, Chen JF, Kuo MC, et al The influence of self-monitoring blood glucose frequency on the oscillation of hemoglobin A1c and chronic complications. Chang Gung Med J. 2012 Jan–Feb;35(1):46–53.
25. Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton AM. Self-monitoring of blood glucose in type 2 diabetes: recent studies. J Diabetes Sci Technol. 2013 Mar 1;7(2):478–88.
26. Austin MM. Self-Monitoring of Blood Glucose in Type 2 Diabetes: Preface. Diabetes Spectrum, 2013;26(2):80–1.
27. Dudley JD. The diabetes educator's role in teaching the diabetic patient. Diabetes Care. 1980 Jan–Feb;3(1):127–33.
28. Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012 Jan 18;1:CD005060.
29. Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, et al. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006 Jul;114(7):384–8.
Andrew Thomas, 1 Medical student, Sree Gokulam Medical College and Research Foundation, Trivandrum, India
Mohan T Shenoy, 2 MBBS, MD, DM, Consultant Endocrinologist, Department of Endocrinology, Sree Gokulam Medical College and Research Foundation, Trivandrum, India
K.T. Shenoy, 3 MBBS, MD, DM, Professor and Head of department, Department of Gastroenterology, Sree Gokulam Medical College and Research Foundation, Trivandrum, India
Nirmal George, 4 MBBS, MD, Assistant Professor, Department of Pharmacology, Sree Gokulam Medical College and Research Foundation, Trivandrum, India
About the Author: Andrew Thomas is currently a final year medical student of Sree Gokulam Medical College and Research Foundation, Trivandrum, India. He is a student reviewer for International Journal of Medical Students and Student British Medical Journal. He is also a recipient of Short-Term Studentship 2019 by ICMR (Indian Council of Medical Research), Best paper award at NATCON 2019, Government medical college, Trivandrum.
Correspondence: Andrew Thomas, Address: Aalamthara - Bhoothamadakki Rd, Venjarammoodu, Kerala 695607, India. Email: drandrewthomasj@gmail.com
Editor: Francisco J. Bonilla-Escobar Student Editors: Diego Carrion Alvarez Student Editors: Brandon Belbeck Student Editors: Adnan Mujanovic Copyeditor: Johnmark Boachie Proofreader: Nikoleta Tellios Layout Editor: Francisco J. Bonilla-Escobar
Cite as: Thomas A, Shenoy MT, Shenoy KT, George N. Glucometers for Patients with Type 2 Diabetes Mellitus: Are they helpful? Int J Med Students. 2021 May-Jun;9(2):140-4
Copyright © 2021 Andrew Thomas, Mohan T Shenoy, K.T. Shenoy, Nirmal George
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 9, NUMBER 2, May-June 2021