Search Results and Exclusion of Articles.
Rebecca Murerwa1, Fidel Gwala2, Thomas Amuti2, Mary Muange3
doi: http://dx.doi.org/10.5195/ijms.2021.850
Volume 10, Number 1: 74-81
Received 21 11 2020: Rev-request 11 01 2021: Rev-request 01 07 2021: Rev-request 03 11 2021: Rev-recd 17 02 2021: Rev-recd 05 07 2021: Rev-recd 11 11 2021: Accepted 14 11 2021
ABSTRACT
Children's exposure to mercurial skin lightening agents at any time during their development, from intra-uterine to early developmental life, can lead to severe detrimental health effects. This is because these skin lightening agents contain inorganic mercury as their active ingredient at varying concentrations that exceed acceptable levels. Mercury does not confer any physiological benefit to the human body, and as such, it has only been linked to numerous adverse effects on users and may pose a possible health risk for children born to, living with, and in contact with skin bleaching agent users. Although studies have shown that inorganic mercury exposure may be detrimental to children, there is a paucity of data, to the best of our knowledge, on reviews exploring specifically the possible routes of exposure to and effects of mercurial skin lightening agents on children. Since prevention is the only key to reducing mercury poisoning and toxicity, this study aims to extensively review the literature on prenatal and postnatal exposure to mercury in children from cosmetic skin lightening agents and discuss possible detrimental effects.
Keywords: Mercury compounds; Inorganic mercury poisoning; Skin lightening preparations; Maternal-fetal exchange; Prenatal exposure delayed effects.
Skin bleaching refers to the process of removing pigment from an individual's skin.1 It is practiced by both men and women in many countries.2-6 Skin lightening agents are widely available as they are sold over the counter in pharmacies and supermarkets.3,7 They are available as gels, creams, and lotions3 and contain various chemicals, including inorganic mercury compounds.4,8 Skin lightening effects are reversible once a person stops using topical skin lightening agents.9 This is because epidermal cells are continuously replaced by new cells capable of melanogenesis.5,6 Topical application of skin lightening agents is often carried out continuously and over a long period to achieve and maintain the desired effects.6 Despite the reversibility of the skin lightening effect, some adverse systemic effects of these agents may persist even after their use is stopped due to the accumulation of harmful components in the user's tissues. Inorganic mercury is the active agent found in mercurial skin lightening agents.10 It replaces copper necessary for tyrosinase enzyme activity, thus inhibiting the synthesis of melanin.10 Human skin is highly permeable to this form of mercury, absorbing 0.8% to 3.7% of the dose applied.11 Once absorbed into the bloodstream, inorganic mercury is transported to various organs such as the liver, ovaries, and kidneys of an individual where, after chronic use, it accumulates and can cause tissue injury and organ dysfunction.6, 12
There is a high prevalence of cosmetic skin lightening among women of reproductive age in African countries with prevalence of 25% in Mali and 30 % in Tanzania.3, 4, 11 This has been attributed to the influence of societal portrayal of lighter individuals as beautiful and the perpetuation of this notion by mass media and popular culture in some areas.8 Furthermore, the use of these agents during pregnancy and lactation has been documented.13,14 The effects of mercurial skin lightening agents on users have been well documented, including skin, renal, and nervous system damage.8, 15-16 These agents also pose a possible health risk for children born to, living with, and in contact with skin bleaching agent users. This study, therefore, aims to extensively review possible prenatal and postnatal exposure to mercury in children from mercurial skin lightening agents, the possible effects associated with such exposure as well as the possible effects through which these effects are mediated.
This is a narrative review. Literature was searched using Google Scholar and PubMed. The keywords entered into PubMed and Google Scholar were “cosmetic skin lightening” or “skin lightening preparations” and “foetal toxicity” or “foetal exposure” or “prenatal exposure” or “prenatal toxicity” and “inorganic mercury”. “Or” and “and” were the operators used. An additional search of the references of selected articles was carried out, and relevant articles were included.
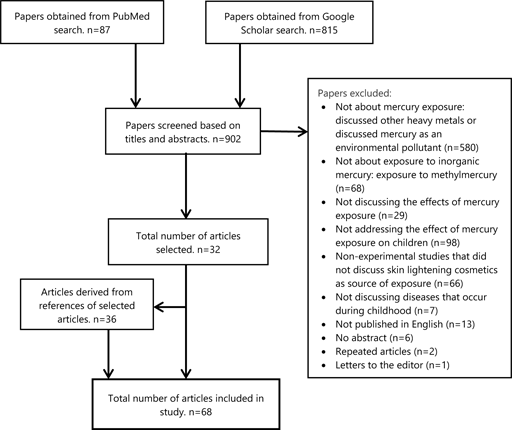
Papers that did not discuss the effects of prenatal and postnatal exposure to inorganic mercury were excluded after screening the titles, abstracts, and reading full texts. Articles published in English were included. Articles that contained the words “skin lightening preparations” or “skin bleaching” were included. Articles that exclusively discussed the effect of methylmercury and mercury exposure following consumption of fish were excluded. Articles that discussed the concentration of mercury in cosmetics without addressing effects on exposed offspring were excluded. The criteria used for inclusion and exclusion are expounded in Figure 1.
Figure 1.Search Results and Exclusion of Articles.

A total of 68 papers were included in this review. The results of the search are summarized in Figure 1. This review included 19 cross-sectional studies, 8 experimental studies, 10 case reports, and 31 reviews following full-text appraisal. Of the cross-sectional studies, three were conducted in Saudi Arabia, 3 in Senegal, 2 in Sweden, 2 in the USA, and 1 in Tanzania, Ghana, Jamaica, Ireland, France, Japan, Canada, Nigeria, and India.
The FDA-approved concentration of mercury in skin lightening products is one part per million (Ppm).17 Mercurial skin lightening agents are documented to have varying mercury concentrations 8,14,18,19, including very high levels of 28000-210000ppm.20 Additionally, mercury is not always listed as an ingredient in these cosmetics, which may contribute to undiscovered cases of exposure.8,21 Cream formulations were found to have the highest concentrations of mercury, and the common practice of mixing different brands in an attempt to increase their potency was shown to increase the likelihood of exposure to very high levels of mercury.8 Application of higher concentrations of mercury can cause more mercury to be absorbed into the bloodstream.
There are three forms of mercury inorganic, organic, and metallic mercury. Of these three, inorganic mercury is added to skin lightening agents because it can penetrate the skin to cause a lightening effect.11 Although it is lipophobic, it accumulates in organs such as the liver and kidneys, causing damage to their cells.22 In these organs, it binds to glutathione and forms mercury-glutathione compounds excreted in bile and urine.23, 24 Additionally, inorganic mercury induces increased expression of metallothionein in renal, hepatic, and placental cells and binds to it.24, 25 By binding in these tissues, mercury accumulates and is removed from the body's circulation. Metallothionein, however, is saturable with increasing doses of mercury, and the ability of these tissues to accumulate mercury may be limited.24 Mercury bound to metallothionein is excreted slowly from the kidney and liver.24 It is also excreted, in smaller amounts, in breast milk, bile, sweat, saliva, and lungs.26
Inorganic mercury in skin lightening agents may vaporize into its elemental/metallic form, which can be absorbed through the alveolar membrane. Once in the bloodstream, lipophilic elemental mercury crosses lipid membranes all over the body.23, 27 Thereafter, it is taken into body tissues, including the red blood cells, liver, and central nervous system cells, and is oxidized by cytosolic catalases to inorganic mercury, which remains trapped in these tissues for a long time owing to its lipophobic nature. 28
The use of skin-lightening agents during pregnancy has been documented.13,29 In a study conducted in Senegal, pregnancy was documented to trigger the practice of skin lightening or to cause an increase in the use of skin lightening agents, especially during the third trimester. 29 This practice may be attributed to physiological hyperpigmentation that accompanies pregnancy, known as chloasma gravidarum. Expectant women who reported using skin lightening agents during pregnancy generally applied these agents all over their bodies except the abdomen.29
Exposure of a pregnant woman to mercury translates to fetal exposure.21, 30 Inorganic mercury is lipophobic and crosses the blood-placental barrier with difficulty, at a lower rate, and to a lesser degree than organic mercury.30 In the placenta, it may induce metallothionein expression in placental cells and bind to it.31 A study showed that the binding of metallothionein and mercury in the placenta reduced the transfer of mercury to the fetus.31 Despite the protective nature of the bond, mercury binding to placental metallothionein may result in harmful changes in the placenta by accumulating in cell membranes and causing impaired membrane fluidity.6 Such changes may impair the transport of essential trace elements like selenium32 amino acids, oxygen, and hormonal production by the placenta, subsequently causing damage to the fetus.31,33
A recent study in which pregnant mice were exposed to inorganic mercury documented that a fraction of inorganic mercury in the placenta may be transported via various transport proteins into fetal tissues.34 Regular and/or chronic exposure of pregnant women to inorganic mercury may result in significant exposure of the fetus.34 The effects of exposure in utero are dependent on the dose.35 Inorganic mercury present in the fetal bloodstream may pose an important health risk to the fetus, especially since there is a trend of higher mercury levels in cord blood compared to maternal blood.30, 36 Oliveira et al34 found that fetal mouse tissues are more susceptible to mercury and suffer from more toxicity than adult mice despite the exposure to the same levels of mercury. This may be attributed to the immaturity of fetal renal systems, which cannot excrete mercury as efficiently as adults can, allowing mercury to accumulate in fetal tissues for more extended periods.30 Such prenatal exposure to mercury, which is nephrotoxic, may result in permanent kidney dysfunction.2
In addition to inorganic mercury absorbed trans-dermally, mercury vapor from mercurial skin lightening agents may be inhaled by expectant mothers.37 In its elemental form, it readily crosses the blood-placental barrier and accumulates in the fetal liver, kidney, and brain.37 The uptake of elemental mercury in the fetus has been shown to increase with increasing gestational age.37 Following transfer to the fetus, elemental mercury can cross the blood-brain barrier of the fetus to cause adverse central nervous system effects.23
Inorganic mercury is excreted in breast milk,26,38 breast milk is preferentially enriched with mercury.39 Bjönberg31 found that the relationship between maternal plasma mercury and breast milk is such that increasing levels of inorganic mercury in maternal plasma led to an increased level of mercury in breast milk. The concentration of mercury in breast milk has been documented in various countries worldwide, with very high concentrations found in Turkey (25.8 µg/L) and Brazil (6.47 µg/L).38 Milk to maternal plasma ratios of 0.6-1 have been documented.31 About 7-15% of ingested inorganic mercury is absorbed.40,41 Breast milk, therefore, is an important route of exposure to inorganic mercury in children.38
Some caregivers have been documented to apply skin-lightening agents onto children's skin.42,43 In addition to this, typical childhood behavior places children in the homes of users at risk of direct contact with the skin lightening agents they may have skin contact with and/or ingest.43 Children have thinner skin than adults and are likely to absorb more mercury into their bloodstream.8 Additional factors that increase the absorption of mercury from skin lightening agents include hydration of skin, higher frequency of application, higher external temperature, and surface area over which the agent is applied.8, 11
Mercury found in skin lightening agents can vaporize to release elemental mercury when applied onto the users' skin and directly from their holding containers.44 In the same study, Copan et al found that mercury vapor levels were very high around bedding and dirty laundry of mercurial cream users. In the same study, mercury vapor levels near jars containing the mercurial compounds in users' homes were documented to range between 12 and 999µg/m3. Mercury vapor is colorless, tasteless, and does not have any distinct scent. Owing to these characteristics, its presence in the home may go unnoticed for a long period. Pregnant women and young children close to users or containers of mercurial skin lightening agents may inhale the vaporized mercury resulting in respiratory tract trauma44 and its absorption through the alveolar membrane.45
Furthermore, mercury vapor is denser than air and settles near the ground where crawling and playing infants are exposed to it.46,47 Children breathe more rapidly than adults and inhale more mercury vapor per body than adults exposed to the same dose.46,48 About 74-80% of the dose of mercury vapor inhaled is absorbed.45 Through this route, skin lightening agents, are potential sources of elemental mercury exposure to users and nonusers in the same household.20
According to the German Human Biomonitoring Commission, no adverse health effects are expected when urinary mercury levels are below 7µg/L in children and women of childbearing age.39 In the same population, urinary mercury levels above 25µg/L are associated with adverse health effects and are levels at which medical practitioners should intervene.
The adverse effects of mercury vary with the levels of exposure and duration of exposure. In terms of inhalation of mercury, the concentration of mercury vapor and the duration of exposure result in variable clinical presentations of toxicity (Table 1).
Table 1.Mercury vapor levels and associated medical relevance.
| Mercury vapor levels | Association |
|---|---|
| 50 µg/m3 | Threshold limit of acceptable mercury vapor levels.23 |
| 0.7-42 µg/m3 (chronic inhalation) | Adverse nervous system effects including impaired cognition sleep disturbance and tremors.32 |
| 1-2 mg/m3 (acute) | Acute mercurial pneumonitis.23 |
In the cell, mercury binds thiol groups in sulfhydryl-containing enzymes resulting in their dysfunction.21,49 Additionally, it interrupts cell membrane ion channels to cause impaired membrane transport.49 It is also a catalyst in the Fenton reaction and may increase cellular production of reactive oxygen species (ROSs), increasing cellular oxidative stress.32 Children are more prone to cellular damage caused by ROSs because they have less developed immune defense against them.38 Al Saleh (2013) showed that reactive oxygen species produced after exposure to inorganic mercury cause oxidative stress. This stress was shown to result in cellular damage in the form of lipid and DNA peroxidation.38 The same study showed that the combined enzyme dysfunction and cell organelle damage caused by mercury accumulation in cells subsequently leads to cellular, tissue, and organ dysfunction.18
Children have higher metabolic rates than adults and rapidly developing organs and organ systems which may be disrupted easily by mercury intoxication.48 Furthermore, children are less efficient at excreting mercury owing to their less mature metabolic processes.48
Elemental mercury can cross the blood-placental and blood-brain barriers.23 Once in the fetal brain, it is oxidized to inorganic mercury, which accumulates in the brain cells owing to its lipophobic nature.23 In a study inorganic mercury was documented to cross the blood-brain barrier of infant mice after exposure in utero.50 In the same study, inorganic mercury was shown to accumulate in the hippocampus. Following exposure of mice to inorganic mercury via skin-lightening cosmetics,51 pathological changes in the brains of exposed mice were documented. These changes included thinning of the cerebral cortex, irregularities of the granular layer of the cortex, and vacuolation in the brainstem and cerebellum. 51 In a study by Chehimi et al.,52 rats exposed to inorganic mercury prenatally had delayed milestones.
Acrodynia is an idiosyncratic hypersensitivity reaction to mercury intoxication.46,53 Some children are more susceptible to developing acrodynia than others- about 1 in every 500 children exposed to mercurial teething creams developed the condition.46 Neurological manifestations of acrodynia in children, including tremor, hypotonia, irritability, apathy, insomnia, and seizures, have been documented.53-55 Inorganic mercury in the central nervous system has been shown to increase susceptibility to seizures and prolong these seizures.56
Mercury has been documented to enter the neuron through calcium and sodium channels and cause permanent depolarization, resulting in neurotransmitter release.57 It, therefore, causes an increase in the release of excitatory neurotransmitters such as glutamate and decreases their uptake from synaptic clefts by astrocytes.58 In addition to this, mercury may cause the decreased synthesis of gamma-Aminobutyric acid (GABA), the main inhibitory neurotransmitter in the nervous system.59 This results in high levels of excitatory neurotransmitters in the extracellular compartment that may cause the over-activation of N-methyl-d-aspartate receptors.58 Owing to the decreased levels of GABA, the excitatory activity of glutamate is unopposed and may cause neurons to enter an excitotoxic cascade.58
Mercury has been documented to cause calcium homeostasis disruption resulting in impaired action potential transmission.58 Additionally, mercury causes inflammation and induces the production of high levels of ROSs by microglia.59 ROSs produced cause mitochondrial dysfunction, lipid and DNA peroxidation, and cause apoptosis and necrosis of neuronal cells.58,59
In neuronal cells, inorganic mercury has been documented to inhibit neuronal cell differentiation by inhibiting the activity of retinoic acid, altering the expression of Microtubule Associated Proteins, and reducing the expression of tubulin βIII needed for polymerization of microtubules.10 Mercury-induced tubulin insufficiency disrupts the scaffolding required for axonal and dendritic formation.59 Subsequently, axons and dendrites collapse and undergo degeneration.59 In these ways, mercury impairs mitosis, disrupts neuronal migration, and is a potent neurotoxin in prenatal and postnatal periods.60
Copan et al44 found that hypertension was a common clinical sign in children exposed to mercury from skin lightening agents and soaps. Mercury inactivates S-adenosyl-methionine causing increased levels of catecholamines.61 The rise in catecholamines may cause a mercury-intoxicated individual to present with tachycardia, hypertension caused by vasoconstriction, hypersalivation, and hyperhidrosis.32 Mercury may also contribute to dysfunctional parasympathetic and sympathetic cardiac control in exposed children.62
It has been documented to cause increased production of ROSs such as superoxide ions.27 ROSs may bind to nitric oxide produced by endothelial cells to form peroxynitrite. Peroxynitrite may cause myocyte cell toxicity and decrease the availability of nitric oxide necessary for vasodilation.27 In addition to causing vasoconstriction via increased sympathetic outflow, mercury may cause reduced vasodilation due to decreased nitric oxide levels and contribute to cases of unexplained hypertension in young age groups.44,62,63
Mercury has been documented to induce autoimmune diseases such as Kawasaki disease in genetically susceptible individuals.55 However, the clinical presentation of Kawasaki disease can resemble acrodynia55 and acrodynia should be considered as a differential.
A young child exposed to mercury vapors, from heating an unknown quantity of mercury, was documented to elevated serum alanine aminotransferase, serum bilirubin, and ornithine carbamoyltransferase following its inhalation.35 Mercury exposure, in this case, was found to cause some degree of hepatic dysfunction. An infant who ingests inorganic mercury in mercuric chloride was documented to present with hepatic enlargement.35
Following the application of mercurial skin lightening agents onto adult mice, inorganic mercury was documented to cause loss of hepatic cells, vacuolation of hepatic cells as well as an increase in Kupffer cells.51 To the best of our knowledge, no studies on specific clinical hepatic dysfunction in children exposed to inorganic mercury have been published.
Gastrointestinal manifestations of acrodynia include salivation, loss of teeth, gum irritation and gingivitis, diarrhea, and anorexia.54 Ingestion of inorganic mercury in the form of mercuric chloride may be highly irritating to the gastrointestinal mucosa and has been documented to cause ulceration and blisters on the lips and tongue of a 19-month-old child.35
Intestinal bacteria exposed to inorganic mercury have been documented to develop resistance to antibiotics.64 Additionally, inorganic mercury that is ingested may be absorbed into gastrointestinal cells and prevent the synthesis and secretion of digestive enzymes such as trypsin, chymotrypsin, and pepsin.18 The above may result in indigestion in children that ingest mercury in breast milk.32
Inorganic mercury is nephrotoxic and children are more susceptible to its effects.38 Following exposure to inorganic mercury in utero, the highest load of mercury is in fetal kidneys.34 The amount of accumulated mercury in the fetal kidneys was positively associated with the dose of mercury to which the pregnant mice were exposed.34
Repeated applications of mercurial skin lightening creams on mice have been documented to accumulate mercury in the kidney and cause nephrotic syndrome.51 Additional pathological effects seen in the kidneys of mice exposed to mercurial compounds in skin lightening creams include focal atrophy of glomerulus, dilatation or obliteration of Bowman's capsule, and vacuolation tubular cells, eosinophilic bodies in proximal tubules, and lymphoid hyperplasia.51 Mercury induced glomerulonephritis has been documented as an autoimmune response to mercury intoxication following exposure to mercurial skin lightening agents and pathologically presents with macrophage and monocyte infiltration.65
In the kidney, inorganic mercury is bound to both glutathione and metallothionein, reducing the amount of mercury circulating in the blood.24 Variations in endogenous levels of the glutathione and metallothionein give rise to variable sensitivity to inorganic mercury and variations in the severity of the extra-renal mercury-induced disease.24 Mercury commonly affects the proximal tubules in the kidney.19 It causes damage to these cells and results in the release of intracellular enzymes such as lysosomal N-acetyl-β-d-glucosaminidase, lactate dehydrogenase, and aspartate aminotransferase.65 The extent of kidney damage due to chronic mercury exposure corresponds to urinary levels of mercury. Levels above 25µg of mercury per Liter of urine are associated with adverse clinical outcomes (Table 2).
Table 2.Urinary mercury levels and their clinical relevance.
| Urinary mercury level (µg of HG/L of urine) | Clinical relevance |
|---|---|
| Less than 7 (HBM I value) | Adverse outcomes are not expected.39 |
| More than 25 (HBM II value) | Adverse outcomes are expected.39 |
| 150 | High likelihood of kidney disease.6 |
Inorganic mercury has been documented to accumulate in the cells of the pituitary glands.66 Luteinizing hormone (LH) produced by the anterior pituitary gland has a sequence of cysteine residues to which mercury has a high affinity.66 Mercury causes dysfunction of LH, resulting in dysfunctional androgen synthesis.66 The androgen imbalance caused by mercury-induced LH dysfunction has been put forward as a possible etiology of autism in mercury-sensitive children exposed to mercury early on in life.66 The accumulation of mercury in the pituitary gland early in neurodevelopment may induce inflammation within the gland.66 The mercury-induced inflammation may impair migration of neural precursors of thyrotrophs and impair their incorporation into the gland, ultimately impairing thyroid gland function.
A recent study documented higher levels of inorganic mercury in cord blood of children born to mothers who continued to carry out skin bleaching during pregnancy.67 These levels of mercury in cord blood were associated with lower fetal Immunoglobulin G levels, which may be associated with increased susceptibility to disease.67 Chronic exposure to inorganic mercury has been documented to exacerbate systemic lupus erythematosus29,47, induce systemic autoimmunity and negatively influence several functions of neutrophils.68
Inorganic mercury applied onto the skin has been documented to cause dermatitis, allergic reactions, and acrodynia in children.18 Acrodynia is an idiosyncratic hypersensitivity reaction occurring in children exposed to inorganic mercury. It presents with cutaneous lesions that include pain, alopecia, swelling of the hands, feet, and nose, desquamation, loss of nails, and, in severe cases, gangrene of fingers and toes.53-55,65
Exposure to mercury early on in life may result in numerous adverse systemic effects in children who are typically more sensitive to its harmful effects than adults. Children's organ systems and tissues are rapidly developing, and damage at these stages may culminate in organ dysfunction that can extend into old age and cause morbidity and mortality later in life.
Many individuals widely use skin lightening creams to achieve lighter skin tones to meet societal standards of beauty and improve the appearance of hyperpigmentation and other skin blemishes. They may, however, pose detrimental health effects to children exposed to them prenatally and in early childhood. Some of these harmful effects include acrodynia, nephrotic syndrome, glomerulonephritis dermatitis, among others. Our findings provide a detailed summary of these harmful effects and their mechanisms and may equip healthcare providers to counsel their at-risk patients appropriately and encourage them to avoid their use during pregnancy.
This review paper brought to our attention that several studies have demonstrated the effects of mercury toxicity on children, but the threshold for toxicity remains poorly elucidated in the literature. As such, we recommend that the toxicity threshold in children be investigated further. In addition to this, the epidemiology of inorganic mercury toxicity, particularly in populations where skin lightening is practiced, is poorly elucidated in the literature. We, therefore, recommend an investigation of the epidemiology of pediatric inorganic mercury toxicity among these populations to gain a complete picture of its burden on child health.
We would like to express our deep gratitude to Doctor Nicholas Mosoba and Anita Wambui Mwaura for proofreading this work.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization, Methodology, Validation, Writing-Original Draft Preparation: RM, FG, TA; Data Curation: RM, FG; Formal Analysis, Investigation, Project Administration: RM; Resources: RM, MM; Supervision: FG, TA, MM; Visualization: RM, FG, TA, MM; Writing-Review and Editing: MM.
1. Charles C. Skin Bleaching, Oppression and Black Resistance Social Science Research Network; 2014 Nov Available from: 10.2139/ssrn.2519822. Last updated January 6 2016; cited August 4 2020.
2. Al-Saleh I, Shinwari N, Al-Amodi M. Accumulation of Mercury in Ovaries of Mice After the Application of Skin-lightening Creams. Biol Trace Elem Res. 2009;131(1):43-54.
3. Lewis K, Robkin N, Gaska K, Njoki L. Investigating Motivations for Women's Skin Bleaching in Tanzania. Psychol Women Q. 2011;35:29-37.
4. Mahe A, Ly F, Aymard G, Dangou JM. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br J Dermatol. 2003;148(3):493-500.
5. Risher JF, De Rosa CT. Inorganic: the other mercury. J Environ Health. 2007;70(4):9-16; discussion 40.
6. Voegborlo RB, Agorku SE, Buabeng-Acheampong B, Zogli E. Total Mercury Content Of Skin Toning Creams And The Potential Risk To The Health Of Women In Ghana. J Sci Technol. 2008
7. Al-Saleh IA. Health implications of mercury exposure in children. Int J Environ Health. 2009;3(1):22-57.
8. Ricketts P, Knight C, Gordon A, Boischio A, Voutchkov M. Mercury Exposure Associated with Use of Skin Lightening Products in Jamaica. J Health Pollut. 2020;10(26):200601.
9. Kain T, Weinstein J, Thompson A, Boggild AK. The “wing-heeled” traveler. Trop Dis Travel Med Vaccines. 2020;6(1):2.
10. Chan TYK, Chan APL, Tang HL. Nephrotic syndrome caused by exposures to skin-lightening cosmetic products containing inorganic mercury. Clin Toxicol. 2020;58(1):9-15.
11. Palmer RB, Godwin DA, McKinney PE. Transdermal Kinetics of A Mercurous Chloride Beauty Cream: An In Vitro Human Skin Analysis. J Toxicol Clin Toxicol. 2000;38(7):701-7.
12. Cullen E, Evans DS, Davidson F, Burke P, Burns D, Flanagan A, et al. Mercury exposure in Ireland: results of the DEMOCOPHES human biomonitoring study. Int J Environ Res Public Health. 2014;11(9):9760-75.
13. AlGhamdi K. The use of topical bleaching agents among women: a cross-sectional study of knowledge, attitude and practices: The use of topical bleaching agents among women. J Eur Acad Dermatol Venereol. 2010;24(10):1214-9.
14. Al-Saleh I. Potential health consequences of applying mercury-containing skin-lightening creams during pregnancy and lactation periods. Int J Hyg Environ Health. 2016;219(4–5):468-74.
15. Boyd AS, Seger D, Vannucci S, Langley M, Abraham JL, King LE. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43(1):81-90.
16. Petit A, Cohen-Ludmann C, Clevenbergh P, Bergmann J-F, Dubertret L. Skin lightening and its complications among African people living in Paris. J Am Acad Dermatol. 2006;55(5):873-8.
17. US Food and Drug Administration . FDA Cosmetics Handbook. Department of Health and Human Services, Public Health Service; 1992.
18. Agrawal SS, Mazhar M. Adulteration of mercury in skin whitening creams – A nephrotoxic agent. Curr Med Res Pract. 2015;5(4):172-5.
19. Sin KW, Tsang HF. Large-scale mercury exposure due to a cream cosmetic: community-wide case series. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2003;9(5):329-34.
20. Copan L, Ujihara A, Jones C, Das R, Kreutzer R, Roisman R, et al. Mercury exposure among household users and nonusers of skin-lightening creams produced in Mexico - California and Virginia, 2010. Morb Mortal Wkly Rep. 2012;61:33-6.
21. Budnik LT, Casteleyn L. Mercury pollution in modern times and its socio-medical consequences. Sci Total Environ. 2019;654:720-34.
22. Martinez-Finley EJ, Aschner M. Recent Advances in Mercury Research. Curr Environ Health Rep. 2014;1(2):163-71.
23. Asano S, Eto K, Kurisaki E, Gunji H, Hiraiwa K, Sato M, et al. Review article: acute inorganic mercury vapor inhalation poisoning. Pathol Int. 2000;50(3):169-74.
24. Tokumoto M, Lee J-Y, Shimada A, Tohyama C, Satoh M. Glutathione has a more important role than metallothionein-I/II against inorganic mercury-induced acute renal toxicity. J Toxicol Sci. 2018;43(4):275-80.
25. Al-Saleh I, Abduljabbar M, Al-Rouqi R, Eltabache C, Al-Rajudi T, Elkhatib R, et al. The extent of mercury (Hg) exposure among Saudi mothers and their respective infants. Environ Monit Assess. 2015;187(11):678.
26. Risher JF; World Health Association and Interantional Programme on Chemical Safety. Elemental mercury and inorganic mercury compounds: human health aspects. Available from https://apps.who.int/iris/handle/10665/42607. Last updated 2003; cited August 9 2020.
27. Fernandes Azevedo B, Barros Furieri L, Peçanha FM, Wiggers GA, Frizera Vassallo P, Ronacher Simões M, et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol. 2012:949048.
28. Davis BJ. Mercury Vapor and Female Reproductive Toxicity. Toxicol Sci. 2001;59(2):291-6.
29. Mahé A, Perret JL, Ly F, Fall F, Rault JP, Dumont A. The cosmetic use of skin-lightening products during pregnancy in Dakar, Senegal: a common and potentially hazardous practice. Trans R Soc Trop Med Hyg. 2007;101(2):183-7.
30. World Health Organization. Children's Exposure to Mercury Compounds . WHO. World Health Organization; 2010 Available from: http://www.who.int/ceh/publications/children_exposure/en/ Last updated 2010; cited August 5 2020.
31. Ask Björnberg K. Mercury exposure during early human development. Institutet för miljömedicin (IMM) / Institute of Enviromental Medicine; 2005. Available from: http://openarchive.ki.se/xmlui/handle/10616/37829. Last updated April 1 2005; cited August 4 2020
32. Rice KM, Walker EM, Wu M, Gillette C, Blough ER. Environmental mercury and its toxic effects. J Prev Med Public Health Yebang Uihakhoe Chi. 2014;47(2):74-83
33. Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect. 2002;110(5):523-6.
34. Oliveira CS, Joshee L, Zalups RK, Pereira ME, Bridges CC. Disposition of inorganic mercury in pregnant rats and their offspring. Toxicology. 2015;335:62-71.
35. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for mercury. Available from: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/1256999. Last updated 1999. Cited August 6 2020.
36. Butler Walker J, Houseman J, Seddon L, McMullen E, Tofflemire K, Mills C, et al. Maternal and umbilical cord blood levels of mercury, lead, cadmium, and essential trace elements in Arctic Canada. Environ Res. 2006;100(3):295-318.
37. Yoshida M. Placental to Fetal Transfer of Mercury and Fetotoxicity. Tohoku J Exp Med. 2002;196(2):79-88.
38. Al-Saleh I, Abduljabbar M, Al-Rouqi R, Elkhatib R, Alshabbaheen A, Shinwari N. Mercury (Hg) Exposure in Breast-Fed Infants and Their Mothers and the Evidence of Oxidative Stress. Biol Trace Elem Res. 2013;153(1–3):145-54.
39. Ruggieri F, Majorani C, Domanico F, Alimonti A. Mercury in Children: Current State on Exposure through Human Biomonitoring Studies. Int J Environ Res Public Health. 2017;14(5):519.
40. Goldman LR, Shannon MW, the Committee on Environmental Health. Technical Report: Mercury in the Environment: Implications for Pediatricians. Pediatrics. 2001;108(1):197-205.
41. Park J-D, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health Yebang Uihakhoe Chi. 2012;45(6):344-52.
42. Darj E, Infanti JJ, Ahlberg BM, Okumu J. “The fairer the better?” Use of potentially toxic skin bleaching products. Afr Health Sci. 2015;15(4):1074-80.
43. Kampalath RA, Jay JA. Sources of Mercury Exposure to Children in Low- and Middle-Income Countries. J Health Pollut. 2015;5(8):33-51.
44. Copan L, Fowles J, Barreau T, McGee N. Mercury Toxicity and Contamination of Households from the Use of Skin Creams Adulterated with Mercurous Chloride (Calomel). Int J Environ Res Public Health. 2015;12(9):10943-54.
45. Oz SG, Tozlu M, Yalcin SS, Sozen T, Guven GS. Mercury vapor inhalation and poisoning of a family. Inhal Toxicol. 2012;24(10):652-8.
46. Lai O, Parsi K, Wu D, Konia T, Younts A, Sinha N, et al. Mercury toxicity presenting as acrodynia and a papulovesicular eruption in a 5-year-old girl. Dermatol Online J. 2016;22(3).
47. Rogers HS, McCullough J, Kieszak S, Caldwell KL, Jones RL, Rubin C. Exposure assessment of young children living in Chicago communities with historic reports of ritualistic use of mercury. Clin Toxicol Phila Pa. 2007;45(3):240-7.
48. Dunn AM, Burns C, Sattler B. Environmental health of children. J Pediatr Health Care. 2003;17(5):223-31.
49. Ozuah PO. Mercury poisoning. Curr Probl Pediatr. 2000 Mar 1;30(3):91-9.
50. Feng W, Wang M, Li B, Liu J, Chai Z, Zhao J, et al. mercury and trace element distribution in organic tissues and regional brain of fetal rat after in utero and weaning exposure to low dose of inorganic mercury. Toxicol Lett. 2004;152(3):223-34.
51. Al-Saleh I, El-Doush I, Shinwari N, Al-Baradei R, Khogali F, Al-Amodi M. Does low mercury containing skin-lightening cream (Fair & Lovely) affect the kidney, liver, and brain of female mice? Cutan Ocul Toxicol. 2005;24(1):11-29.
52. Chehimi L, Roy V, Jeljeli M, Sakly M. Chronic exposure to mercuric chloride during gestation affects sensorimotor development and later behaviour in rats. Behav Brain Res. 2012;234(1):43-50.
53. Abbaslou P, Zaman T. A child with elemental mercury poisoning and unusual brain MRI findings. Clin Toxicol. 2006;44(1):85-8.
54. De Bont B, Lauwerys R, Govaerts H, Moulin D. Yellow mercuric oxide ointment and mercury intoxication. Eur J Pediatr. 1986;145(3):217-8.
55. Guzzi G, Pigatto PD. Metal Allergy: Mercury. In: Chen JK, Thyssen JP, editors. Metal Allergy: From Dermatitis to Implant and Device Failure [Internet]. Cham: Springer International Publishing; 2018 [cited 2020 Aug 6]. p. 397-421. Available from: https://doi.org/10.1007/978-3-319-58503-1_31
56. Szász A, Barna B, Gajda Z, Galbács G, Kirsch-Volders M, Szente M. Effects of continuous low-dose exposure to organic and inorganic mercury during development on epileptogenicity in rats. Neurotoxicology. 2002;23(2):197-206.
57. World Health Organization, International Programme on Chemical Safety (IPCS). Inorganic mercury. WHO; 1991. Available from: https://apps.who.int/iris/handle/10665/40626. cited 2020 Aug 6.
58. Wallace DR, Lienemann E, Hood AN. Clinical aspects of mercury neurotoxicity. In: Clinical Neurotoxicology. Elsevier; 2009 [cited 2020 Aug 5]. p. 251-8. Available from: https://linkinghub.elsevier.com/retrieve/pii/C20090375686
59. Kern JK, Geier DA, Audhya T, King PG, Sykes LK, Geier MR. Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiol Exp (Warsz). 2012;72(2):113-53.
60. Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol. 2003;18(3):149-75.
61. Baum CR. Mercury: What's In It For Kids? Clin Pediatr Emerg Med. 2012;13(4):324-30.
62. Bose-O'Reilly S, McCarty KM, Steckling N, Lettmeier B. Mercury Exposure and Children's Health. Curr Probl Pediatr Adolesc Health Care. 2010;40(8):186-215.
63. Wössmann W, Kohl M, Grüning G, Bucsky P. Mercury intoxication presenting with hypertension and tachycardia. Arch Dis Child. 1999;80(6):556-7.
64. Summers AO, Wireman J, Vimy MJ, Lorscheider FL, Marshall B, Levy SB, et al. mercury released from dental “silver” fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother. 1993;37(4):825-34.
65. Gardner R, Nyland J. Immunotoxic Effects of Mercury. In 2016. p. 273-302.
66. Laks DR. Luteinizing hormone provides a causal mechanism for mercury associated disease. Med Hypotheses. 2010;74(4):698-701.
67. Obiageli AN. Immunoglobulin levels in maternal blood, cord blood and breast milk of Nigerian pregnant women using hydroquinone and non-hydroquinone containing skin lightening creams. Our Dermatol Online. 2019;10(2):131-7.
68. Pollard KM, Cauvi DM, Toomey CB, Hultman P, Kono DH. Mercury-induced inflammation and autoimmunity. Biochim Biophys Acta Gen Subj. 2019;1863(12):129299.
Rebecca Murerwa, 1 Medical Student. University of Nairobi. Research in Medicine Kenya (ReMed Kenya), Nairobi, Kenya.
Fidel Gwala, 2 BSc Anatomy. University of Nairobi. Research in Medicine Kenya (ReMed Kenya), Nairobi, Kenya.
Thomas Amuti, 2 BSc Anatomy. University of Nairobi. Research in Medicine Kenya (ReMed Kenya), Nairobi, Kenya.
Mary Muange, 3 MBChB, MMed, Pediatric Consultant. Kangundo Sub-county Hospital, Kangundo Town, Machakos County, Kenya.
About the Author: Rebecca Murerwa is a final year medical student at the University of Nairobi, Nairobi, Kenya of a six-year program. She has published research on the morphological variations of the calcaneus in the Kenyan population.
Correspondence: Rebecca Murerwa. Address: University of Nairobi, Kenya. Email: rebeccamurerwa@gmail.com
Editor: Francisco J. Bonilla-Escobar
Student editor: Adnan Mujanovic, Benjamin Liu, Abdul Basith K M, Johnmark Boachie,
& Joseph Tonge
Copyeditor: Nguyen Tran Minh Duc
Proofreader: Sebastian Diebel
Layout Editor: Lucianne Adhiambo Odiero
Process: Peer-reviewed
Cite as: Murerwa R, Gwala F, Amuti T, Muange M. Childhood effects of prenatal and postnatal exposure to mercurial skin lightening agents. Literature Review. Int J Med Stud. 2022 Jan-Mar;10(1):74-81.
Copyright © 2022 Rebecca Murerwa, Fidel Gwala, Thomas Amuti, Mary Muange
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 10, NUMBER 1, Jan-Mar 2022