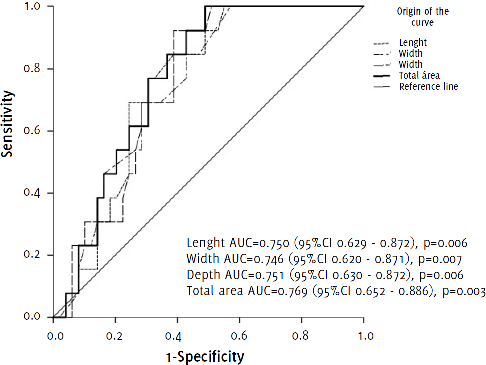
Receiver operator curve of arteriovenous malformation size as a predictor for mortality.
Patricio García-Espinosa1, Edgar Botello-Hernández1, Gabriela Torres-Hernández1, Clarissa Guerrero-Cavazos1, Estefania Villareal-Garza2, Andrea Flores-Rodriguez1
doi: http://dx.doi.org/10.5195/ijms.2021.884
Volume 9, Number 3: 213-218
Received 24 12 2020: Rev-request 09 04 2021: Rev-request 02 06 2021: Rev-request 07 07 2021: Rev-request 24 08 2021: Rev-recd 28 04 2021: Rev-recd 02 06 2021: Rev-recd 10 08 2021: Rev-recd 24 08 2021: Accepted 24 08 2021: Publication 31 08 2021
ABSTRACT
Abstract Background:Arteriovenous Malformations (AVMs) are abnormalities in intracranial vessels between the arterial and venous systems. This study aimed to identify the predictors of mortality in patients that presented to our hospital with AVMs, ruptured or unruptured, and correlate them to those available in the literature.
Methods:An analytical, observational, retrospective study was performed to review data of patients with cerebral AVMs in the University Hospital “Dr José Eleuterio González” from January 2016 to December 2020. Clinical files were reviewed based on AVMs diagnosis according to the International Classification of Diseases 10th Revision, ICD-10. Variables were subjected to a univariate analysis and those found significant (p-value < 0.05) were subjected to a logistic regression.
Results:A total of 80 patients were included in our study. Most of the participants were females (56.3%) and three were pregnant. The most common presenting symptom was holocranial headache (34 cases) occurring between the hours of 22:00 to 7:00. The most significant predictors of mortality were a total bleeding volume greater than 9.18 cm3 (p = 0.010), the presence of more than one symptom (p = 0.041), and a history of previous cerebral intraparenchymal hemorrhage (p = 0.014).
Conclusion:Results demonstrated an important association between intracranial bleeding and mortality. Ultimately, more prospective studies are needed to determine predictor factors for mortality in AVMs patients.
Keywords: Arteriovenous Malformation; Cerebral Hemorrhage; Intracranial Hemorrhages; Nervous System Malformation (Source: MeSH-NLM).
Arteriovenous Malformations (AVMs) are abnormalities in intracranial vessels between the arterial and venous systems.1 They consist of abnormal dilation of vascular structure, forming a nest between the two systems without capillaries, causing arterial blood to reach the venous system.2 In turn, the formed nest has its own circulation system, formed by its own arteries (referred to as vasa-vasorum); however, unlike healthy blood vessels, these lack normal innervation and therefore lack the ability to self-regulate arterial flow within the nest.3
Although in adults AVMs are regarded as acquired malformations, in infants they are believed to be congenital.4 The pathogenesis of congenital AVMs is poorly understood. It is suggested that they develop during the embryogenesis of the primordial vascular system between the third and twelth week of gestation, and are therefore present at birth.4 The most common clinical presentation of cerebral AVMs in pediatric patients is sudden rupture. The first signs and symptoms are usually those associated with intracerebral hemorrhage in 41-79% of cases.5 We have not come across any epidemiological study to determine the most common cause of AVM onset in Mexican pediatric patients.
The average age of AVM appearance is between the ages of 20 and 40, without specific gender prevalence.6 Despite being more common in adults, the probability of a rupture is comparatively lower in the adult population than in the pediatric one. However, rupture and consequent hemorrhage are still the most common cause of symptoms onset.6 When left untreated, the probability of rupture ranges between 2.10–4.12%.7 There is a suggested association between hormonal changes in puberty and AVM rupture, which is why it is believed that the hormonal changes of pregnancy could also represent a period of increased rupture risk.6
The most important classification for cerebral AVMs was described by Spetzler and Martin in 1986, which is used to determine the most appropriate therapeutic approach for these patients.10 The Spetzler Martin Grading Scale ranges from I to VI, where Grade I represents a small, superficial malformation in a non-eloquent portion of the cortex, Grade V represents a large, deep malformation in an eloquent portion of the cortex, and Grade VI is inoperable. For the diagnosis and visualization of AVMs, angiography is considered to be the gold standard because it gives a visualization of the pathology that allows for both diagnostics and treatment planning.11,12 Initial visualization is intended to be non-invasive, for which Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) are often used, especially in patients with diffuse or non-specific symptoms such as seizures or headaches. A CT scan is considered superior to MRI for observing vasculature, however, an MRI is a better choice for visualizing adjacent structures and planning treatment.13–15
Therapeutic management includes different modalities of surgical resection, endovascular embolization, and stereotactic surgery (particularly in those with a nest size smaller than 3 cm at the largest diameter).16 Currently attempts have been made to develop a noninvasive medical treatment, or a “wait-and-see” treatment approach, with little to no recovery period, for patients with unruptured AVM.17,18
Current literature on this subject is not specific to the Mexican community or even the population of Centarl/South America in general. Due to the socio-demographic variants of this population, which are far from the economically developed societies of Northern America and Europe where most AVM studies have been performed, the literature cannot fully be generalized for the adult or pediatric AVM population of Mexico. In the current AVM literature, variables such as previous history of ruptured AVMs, pattern of venous drainage, and the location of the AVM itself have been found to be significant, however, these risk factors have been proposed mostly by singlecenter studies, and have not been always replicated, as highlighted by Rutledge WC, et al in 2014.19 In addition, although some data is available in the Hispanic literature, they are of suboptimal quality, as they represent a low number of cases and do not show the results of all departments that may be involved in the review of ruptured AVMs.6,14 For this reason, the present study reviewed the experience of a single, tertiary-care centre in Mexico to seek a representation of the present phenomenon, to try to replicate results from the literature, and to give rise to further research in treating AVMs in the field with the objective of generating AVM epidemiological datasets from which predictors of mortalities could be extracted, among the Latin-American population.
This was an analytical, observational, retrospective study. A review of the clinical records for each participant was carried out by senior medical students. We included patient data from the last five years because that is the maximum time a patient's file is kept after their hospitalization. Finally, an Excel database was created in order to extract relevant data using an Excel processor. Consent for publication and ethical approval came from the Ethical and Research Commitment from Hospital Universitario “Dr. José Eleuterio González” at Universidad Autónoma de Nuevo León (UANL) Institute, with the approval code NR18-0002.
We reviewed available data of patients who were hospitalized at the UANL, University Hospital from January 2016 to December 2020 for cerebral AVMs, regardless of whether their malformation had ruptured. The data was provided by the statistics department of our institution and the clinical files and CT images were reviewed by a neurology resident. In order to obtain all the existing patient data from our institution, patient files were identified using the following codes of the tenth revision of International Classification of Diseases (ICD-10): Q27, Q28, I60, I62 and I69 – with our main focus on Q28.20
The sample was taken by convenience method of sampling since the studied sample was the total number patients who visited our center in the last five years. We included all patients from every department or division within our hospital who were diagnosed with a cerebral AVM. We excluded patients who were found not have a cerebral AVM and whose pathology-oriented medical assessment revealed a differential diagnosis such as cavernous angioma or capillary telangiectasias, among others.
Clinical and demographic data obtained included sex, age, time of symptoms evolution, treatment details, imaging findings, all other predictive factors that were previously known, and comorbidities. In addition, data extracted from patient registries included treatment outcome, subsequent or previous rupture at the time of diagnosis, number of hospitalization days, and time and place of rupture. The population studied was divided into those with a rupture and those without a rupture of an AVM at the moment of arrival in our institution.
For the analysis, IBM SPSS Software 23.0 version,21 RStudio version 4.0.2 (2020-06-22), and ggplot2 package were used. To compare the data between the groups, Student's-t test and Chi-squared test were used depending on the type of variable, T-test on variables with normal distribution and Chi-squared test on independent random variables with standard normal distribution. For correlations, Pearson's correlation coefficient was reported. A p-value <0.05 was considered statistically significant. Variables that resulted in statistically significant value in univariate analysis were subjected to a logistic regression model.
Using the ICD-10 codes, a total of 486 files were obtained. After applying our inclusion and exclusion criteria, we included 80 patient files for subsequent analysis. The average age of our patients was 26.9 ± 17.5 years, and just over half of the population was female N=45 (56.3%), with three (n=3) of them being pregnant at the time of their first consultation.
Most common presenting symptom was holocranial headache, foundin 34 (23.9%) patients, followed by a generalized tonic-clonic seizure in 29 (20.4%) patients, and lastly a loss of consciousness in 24 (16.9%) patients. Only 6 (7.5%) patients presented without any symptoms, and 1 (1.25%) patient had attended a scheduled consultation at the neurosurgery facility. A total of 47 (58.8%) patients presented with active bleeding from AVM rupture and a holocranial headache was the most common symptom reported, being present in 12 (25.7%) of these cases, followed by altered state of consciousness in 10 (21.2%) patients. The onset of symptomsoccurred between the hours of 22:00 to 7:00 in the majority of patients (61.3%), which are the hours that are regularly not dedicated to work or school in our country. At symptom onset, most patients were asleep, resting at home, or waking up. (Table 1)
Table 1.General characteristics population.
| Characteristic | n (%) |
|---|---|
| Female gender | 45 (56.3) |
| Age in years† | 26.9 ± 17.5 |
| Symptomatology | |
| Holocranial headache | 34 (23.9) |
| Tonic-Clonic Seizures | 29 (20.4) |
| Loss of Consciousness | 24 (16.9) |
| Hemibody paralysis | 10 (7.0) |
| Vomiting | 9 (6.3) |
| Asymptomatic | 6 (4.2) |
| Hemicranial headache | 5 (3.5) |
| Aphasia | 4 (2.8) |
| Other | 21 (14.8) |
| Comorbidities and Predictors | |
| Diabetes | 15 (18.8) |
| Hypertension | 14 (17.5) |
| Cancer | 2 (2.5) |
| BMI | |
| Obesity (> 30) | 37 (46.3) |
| Overweight (25 – 29.9) | 32 (40.0) |
| Normal weight (18.5 – 24.9) | 11 (13.8) |
| Smoker | 32 (40.0) |
| Sedentary lifestyle | 36 (45.0) |
| Pregnancy | 3 (3.8) |
| Event characteristics | |
| Temporality | |
| 22:00 – 7:00 | 49 (61.3) |
| 7:01 – 21:59 | 31 (38.8) |
| Place or action of the patient | |
| Asleep | 22 (27.5) |
| House | 16 (20.0) |
| Waking up | 12 (15.0) |
| Elective consultation | 11 (13.8) |
| Working, school | 9 (11.3) |
| Showering | 6 (7.5) |
| Exercising | 3 (3.8) |
| Street | 1 (1.3) |
| Hemorrhage | |
| All-age hemorrhage | 47/80 (58.8) |
| Pediatric hemorrhage (0–17 years) | 24/30 (80.0) |
| Adult hemorrhage (.18 years) | 23/50 (46.0) |
| Previous hemorrhage | 23 (28.7) |
| Posterior hemorrhage | 10 (12.5) |
| Treatment used | |
| Angio-embolism | 41 (39.8) |
| Surgical excision by craniotomy | 37 (35.9) |
| Radiosurgery | 13 (12.6) |
| Conservative management | 12 (11.7) |
| Mortality | |
| All-age mortality | 14/80 (17.5) |
| Pediatric mortality (0–17 years) | 10/30 (33.3) |
| Mortality in adults (.18 years) | 4/50 (8.0) |
Among comorbidities and predictive factors, the most common were the existence of previous cerebral intraparenchymal hemorrhage observed in 23 (28.7%) patients, followed by Type 2 Diabetes Mellitus as the second most common comorbidity in 15 (18.8%) patients, and Systemic Arterial Hypertension in third place in 14 (17.5%) patients. Inquiring more about personal history, we found that almost half of our included patients led a sedentary lifestyle or were smokers (45% and 40%, respectively).
The median AVM size was 10.29 cm3 (2.49 cm3 - 35.66 cm3). Forty-one (51.2%) had AVM on their right side, mainly found in the frontal lobe in 24 (30%) patients. The middle cerebral artery was the most common nutrient artery in 34 (32.1%) patients. In the venous system, the collateral veins of the superior sagittal sinus in 21 (20.8%) patients were the most commonly affected ones. For diagnosis and classification, CT scans were performed. For more detailed characteristics, consult Table 2.
Table 2.Characteristics of arteriovenous malformations
| Characteristic | n (%) |
|---|---|
| Laterality (n=80) | |
| Right | 41 (51.2) |
| Left | 37 (46.3) |
| Bilateral | 2 (2.5) |
| Cerebral location (n=80) | |
| Frontal | 24 (30.0) |
| Occipital | 15 (18.8) |
| Temporary | 12 (15.0) |
| Frontoparietal | 8 (10.0) |
| Frontotemporal | 6 (7.5) |
| Parietal | 3 (3.8) |
| Cerebellum | 3 (3.8) |
| Other | 9 (11.3) |
| Nutritional arteries (n=106) | |
| Deep middle cerebral artery | 34 (32.1) |
| Deep posterior cerebral artery | 28 (26.4) |
| Deep anterior cerebral artery | 27 (25.5) |
| Posterior inferior cerebellar artery | 8 (7.5) |
| Internal carotid artery | 8 (7.5) |
| Middle meningeal artery | 1 (0.9) |
| Venous drainage (n=101) | |
| Collaterals of the superior sagittal sinus | 21 (20.8) |
| Vein of Galen | 14 (13.9) |
| Deep circulation | 10 (9.9) |
| Superior longitudinal sinus | 8 (7.9) |
| Internal cerebral vein | 8 (7.9) |
| Superficial cerebral veins | 8 (7.9) |
| Other | 32 (31.7) |
| Spetzler-Martin arteriovenous malformation grading system | |
| I | 7 (8.8) |
| II | 37 (46.3) |
| III | 19 (23.8) |
| IV–VI | 17 (21.3) |
For treatment, angio-embolism alone, or accompanied by radiosurgery or surgical excision by craniotomy, were the most common treatment options (41 patients, 39.8%), followed by craniotomy in 37 (35.92%) and radiosurgery in 13 (12.6%) patients. A conservative approach was chosen by 12 (11.7%) patients, showing that the vast majority decided not to resort to the “wait-and-see” treatment (Table 1).
Figure 1 illustrates AVMs size as a predictor of mortality. A total volume in cubic centimeters greater than 9.18 cm3 (OR 0.063 (0.008 – 0.519)), as well as the presence of more than one symptom (OR 4.022 (1.026 – 15.467)), and a history of previous cerebral intraparenchymal hemorrhage (OR 4.533 (1.359 – 15.126)), were significant predictors factors for mortality (p< 0.05), as shown in Table 3.
Figure 1.Receiver operator curve of arteriovenous malformation size as a predictor for mortality.

Odds ratio of the analyzed variables as predictors of mortality
| Characteristic | Odds ratio (95% Confidence interval) | p-value |
|---|---|---|
| Arteriovenous Malformation Size | ||
| Total area (cm3) | ||
| ≤9.18 cm | 0.063 (0.008 – 0.519) | 0.01 |
| >9.18 cm | 16.00 (1.926 – 132–899) | 0.01 |
| Length | ||
| ≤3.36 cm | 0.150 (0.042 – 0.539) | 0.004 |
| >3.36 cm | 6,667 (1,854 – 23,973) | 0.004 |
| Width | ||
| ≤1.92 cm | 0.077 (0.010 – 0.622) | 0.016 |
| >1.92 cm | 13.00 (1.607 – 105.146) | 0.016 |
| Depth | ||
| ≤1.77 cm | 0.053 (0.006 – 0.439) | 0.007 |
| >1.77 cm | 18,947 (2,276 – 157–757) | 0.007 |
| Number of symptoms | ||
| ≤1 symptom | 0.242 (0.062 – 0.946) | 0.041 |
| >1 symptom | 4,022 (1,026 – 15,467) | 0.041 |
| Hemorrhage | ||
| Previous hemorrhage | 4,533 (1,359 – 15,126) | 0.014 |
| Current hemorrhage | 12,235 (1,513 – 98,966) | 0.019 |
| Posterior hemorrhage | 2,299 (0.514 – 10.280) | 0.276 |
| Laterality | ||
| Left | 1,192 (0.579 – 6.318) | 0.288 |
| Right | 0.590 (0.179 – 1.950) | 0.387 |
| Bilateral | 5.33 (0.683 – 41.622) | 0.11 |
| Age | ||
| ≤ 17 years | 5,750 (1,610 – 20,553) | 0.007 |
| ≥18 years | 0.174 (0.049 – 0.621) | 0.007 |
| Sex | ||
| Female | 0.737 (0.232 – 2.341) | 0.605 |
| Male | 1.357 (0.427 – 4.311) | 0.605 |
This analytical, retrospective study analyzed AVM mortality risk factors in 80 patient files from University Hospital “Dr José Eleuterio González” from January 2016 to December 2020.
We found that the average age of AVM presentation was 26.8 years, with a slightly higher prevalence among the female sex. Obesity, Type 2 Diabetes Mellitus, Systemic Arterial Hypertension, and sedentary lifestyle were present in a substantial part of the study cohort. The most significant predictors of mortality were a total volume greater than 9.18 cm3, the presence of more than one symptom, and a history of previous cerebral intraparenchymal hemorrhage. The most common presenting symptom was a holocranial headache occurring between the hours of 22:00 to 7:00. The timing of symptomatology presentation, as a predictor of mortality, could be one of the most important findings of the present study.
Our findings differ from those provided by the National Hospital of Neurology and Neurosurgery of Mexico, which report an average presenting age of 32.9 years and no gender predilection.22 We also found that holocranial headache was the most common presenting symptom which conicides with what was previously reported by Mariano Rinaldi in 2015.23 Rinaldi not only found that holocranial headache was most commonly reported at symptom onset, but that 30.8% of patients were classified as Spetzler-Martin class III. We found that 23.8% of our patients were a class III Spetzler-Martin. It has been reported that grades I and II have good results with microsurgery, while grades III and IV mostly benefit from endovascular embolism.23 In our center, 68 (85%) subjects had a surgical approach, and at least 23 (33.8%) shared an approach through craniotomy and/or radiosurgery in addition to angio-embolism. These approaches are in accordance with AHA 2017 recommendations for AVMs Spetzler-Martin III and IV.24
We found other similarities to Rinaldi's previously reported results, such as the distribution of AVMs towards the right side and mainly in the frontal lobe, in 51.2% and 30% of cases, respectively. Another important factor in this context was the patient's history of past cerebral intraparenchymal haemorrhage (28.7%), as it is one of the variables that has been most represented in the literature as a predictor of mortality in bleeding AVMs, which our results replicate.23
An interesting finding in our study was regarding pregnant women. According to what has been reported in the literature, it was found that, although rare, AVMs are responsible for about 50% of subarachnoid hemorrhages in pregnant women and are the third cause of non-obstetric maternal mortality. However, there are discrepancies in the literature regarding the influence of pregnancy on the natural history of AVM. 25,26,27 We did not find a higher mortality risk for pregnant patients in our study. Although it should be noted that the number of included pregnant patients in our study was small.
Overall mortality in our sample of 80 patients was 17.5% (14 patients, 10 pediatrics and 4 adults). The pediatric mortality was 33.3% (10 patients out of 30). The adult mortality was only 8% (4 patients out of 50). The mortality in the adult population is similar to those reported by Hillman, et al.28 and Rinaldi et al.23, who reported a 13.46% mortality rate. Other studies also reported a moratliy rate ranging from 10 to 15%.17,22 However, the mortality in the pediatric population is vastly different from that of the literature, being 33.3% in our study and 12% in a study published by Riordan, et al.29
Finally, hemorrhage is an important factor when it comes to mortality, which was the most common presentation in our study together with its associated symptomatology, the holocranial headache. Hemorrhage occured in 24 out of 30 pediatric patients, accounting for a pediatric hemorrhage mortality of 42%. While in adults hemorrhage was present in only 23 out of 50 patiens, with a mortality rate of about 17%. High rates of bleeding in pediatrics could explain their high mortality rates. While in adults, the mortality in ruptured AVMs does approach the 10-20% ratio in our study, which is consistent with what other authors have reported. 30,31
Interestingly, the chronobiology of AVM's rupture is not actually described in the literature, but it has been seen that in children the onset of symptoms can generally be found in the morning hours, regardless of their daily activities and waking up time.32,33,34 The time of symptomatology presentation has been highlighted as a poor prognostic factor in both children and adults in our study, without being demonstrated by the literature.32–38 We found a high prevalence of symptom onset in non-working hours, warranting a more specific analysis focusing on this parameter.
The main limitation of this study is that it is retrospective in nature. More ambispective and prospective studies are necessary in order to identify other potential predicting factors. Another limitation is that, due to the patient privacy rules of our hospital, patient files can only be kept for five years from their last visit, and thus no information was available for previous years. Finally, it is possible that not all AVMs were documented, since analysed clinical records do not always contain reliable and complete data.
In this study we presented the relationship between mortality and age. Despite the fact that AVMs are more common in adults, they have a lower mortality when compared to children. Because there is no research comparing paediatric and adult populations on this topic in Mexico, and few studies focused on AVMs in general, it is critical to underline the significance of this disease, which is considered silent but lethal when active bleeding occurs. Identifying epidemiological characteristics of the country's main tertiary care centres could serve as a springboard for more targeted and beneficial studies into the pathogenesis, prevention, and treatment of AVMs.
GECEN and GENEP groups of medical students. Ethical and Investigation Commitment of Medicine School who approves this originial research.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Conceptualization: PGE, GTH & CGC. Data Curation: GTH & AFR. Formal Analysis: PGE, EBH, GTH & AFR. Funding Acquisition: PGE & AFR. Investigation: PGE, EBH, CGC & EVG. Methodology: EBH, CGC & EVG. Project Administration, & Supervision: PGE, EBH & AFR. Resources, Software, & Validation: EBH, GTH & AFR. Visualization, & Writing – Original Draft Preparation: CGC & EVG. Writing – Review & Editing: PGE, EBH, GTH, CGC, EVG & AFR.
1. Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R. Cerebral arteriovenous malformations: evaluation and management. ScientificWorldJournal. 2014 Oct 15;2014:649036–.
2. Tranvinh E, Heit JJ, Hacein-Bey L, Provenzale J, Wintermark M. Contemporary imaging of cerebral arteriovenous malformations. Am J Roentgenol. 2017 Jun;208(6):1320–30.
3. Chen C-J, Ding D, Derdeyn CP, Lanzino G, Friedlander RM, Southerland AM, et al. Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology. 2020 Nov 17;95(20):917–27.
4. Komiyama M. Pathogenesis of Brain Arteriovenous Malformations. Neurol Med Chir (Tokyo). 2016 Jun 15;56(6):317–25.
5. StatPearls Publishing. Arteriovenous Malformation (AVM) Of The Brain. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430744/. Last updated Jun 30, 2020; cited Jan 10, 2021.
6. Di Rocco C, Tamburrini G, Rollo M. Cerebral arteriovenous malformations in children. Acta Neurochir (Wien). 2000 Feb;142(2):145–8.
7. Hofmeister C, Stapf C, Hartmann A, Sciacca RR, Mansmann U, terBrugge K, et al. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke. 2000 Jun 1;31(6):1307–10.
8. Abecassis IJ, Xu DS, Batjer HH, Bendok BR. Natural history of brain arteriovenous malformations: a systematic review. Neurosurg Focus. 2014 Sep;37(3):E7.
9. El-Ghanem M, Kass-Hout T, Kass-Hout O, Alderazi YJ, Amuluru K, Al-Mufti F, et al. Arteriovenous Malformations in the Pediatric Population: Review of the Existing Literature. Interv Neurol. 2016 Sep;5(3–4):218–25.
10. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986 Oct;65(4):476–83.
11. Turjman F, Massoud TF, Viñuela F, Sayre JW, Guglielmi G, Duckwiler G. Aneurysms related to cerebral arteriovenous malformations: superselective angiographic assessment in 58 patients. AJNR Am J Neuroradiol. 1994 Oct;15(9):1601–5.
12. Mossa-Basha M, Chen J, Gandhi D. Imaging of cerebral arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012 Jan;23(1):27–42.
13. Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007 Jun 28;356(26):2704–12.
14. Brown RDJ, Flemming KD, Meyer FB, Cloft HJ, Pollock BE, Link ML. Natural history, evaluation, and management of intracranial vascular malformations. Mayo Clin Proc. 2005 Feb 1;80(2):269–81.
15. Choi JH, Mohr JP. Brain arteriovenous malformations in adults. Lancet Neurol. 2005 May 1;4(5):299–308.
16. Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg. 2002 Jan;96(1):79–85.
17. Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet (London, England). 2014 Feb 15;383(9917):614–21.
18. Elhammady MS, Heros RC. the ARUBA study: where do we go from here? J Neurosurg. 2017 Feb;126(2):481–5.
19. Rutledge WC, Ko NU, Lawton MT, Kim H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl Stroke Res. 2014 Oct;5(5):538–42.
20. World Health Organization. Chapter XVII: Congenital malformations, deformations and chromosomal abnormalities. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Avaible from: https://icd.who.int/browse10/2019/en. Last updated 2019; cited Sep 01, 2021.
21. IBM Corp. (2015). IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.
22. Rodríguez-Parra V, Aburto-Murrieta Y, Zenteno-Castellanos MA. [ Description of clinical and angiographic factors associated with hemorrhage in cerebral arteriovenous malformations treated with embolization]. Arch Neurocien. 2010 Oct;15(4):211–6. Spanish
23. Rinaldi M, Mezzano E, Berra MS, Parés HR, Olocco RV, Papalini FR. Arteriovenous Malformations - checking and descriptive analysis of 52 AVMs treated for the 2000-2010 period. Surg Neurol Int. 2015 Oct 12;6(Suppl 20):S511–S23.
24. Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP, et al. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017 Jun 22;48(8):e200–24.
25. Carvalho CS, Resende F, Centeno MJ, Ribeiro I, Moreira J. [Anesthetic Approach of Pregnant Woman with Cerebral Arteriovenous Malformation and Subarachnoid Hemorrhage during Pregnancy: Case Report]. Brazilian J Anesthesiol. 2013 Mar-Apr;63(2):223–6. Spanish
26. Goya MM, Plasencia WM, Domingo J, Arencibia A, Barber MA, García-Hernández JA. [Intracranial hemorrhage associated with arteriovenous malformations]. Clin Invest Ginecol Obstet. 200431(10):370–5. Spanish
27. Vega-Basulto SD, Lafontaine-Terry E, Gutiérrez-Muñoz FG, Roura-Carrasco J, Pardo-Camacho G. [Intracranial hemorrhage due to aneurysms and arteriovenous malformations during pregnancy and puerperium. Neurosurgery]. 200819(1):25–34. Spanish
28. Hillman J. Population-based analysis of arteriovenous malformation treatment. J Neurosurg. 2001 Oct;95(4):633–7.
29. Riordan CP, Orbach DB, Smith ER, Scott RM. Acute fatal hemorrhage from previously undiagnosed cerebral arteriovenous malformations in children: a single-center experience. J Neurosurg Pediatr. 2018 Sep;22(3):244–50.
30. Sahlein DH, Mora P, Becske T, Huang P, Jafar JJ, Connolly ES, et al. Features predictive of brain arteriovenous malformation hemorrhage: extrapolation to a physiologic model. Stroke. 2014 Jul 12;45(7):1964–70.
31. Schramm J, Schaller K, Esche J, Boström A. Microsurgery for cerebral arteriovenous malformations: subgroup outcomes in a consecutive series of 288 cases. J Neurosurg. 2017 Apr;126(4):1056–63.
32. Xie DX, Dedmon MM, O'Connell BP, He LL, Wellons III JC, Rivas A. Surgical management of a hemorrhagic pediatric brainstem cavernous malformation–A case report. Otolaryngol Case Reports. 20173:7–9.
33. Riordan CP, Orbach DB, Smith ER, Scott RM. Acute fatal hemorrhage from previously undiagnosed cerebral arteriovenous malformations in children: a single-center experience. J Neurosurg Pediatr. 2018 Sep;22(3):244–50.
34. Tascu A, Pascal C, Florea SM, Mircea S. Spontaneous intracranial hemorrhage in children –ruptured lobar arteriovenous malformations: report of two cases. Romanian Neurosurgery. 2015 Mar 15;22(1):85–92.
35. Pezeshkpour P, Dmytriw AA, Phan K, Shroff MM, Dirks P, Kulkarni A V, et al. Treatment strategies and related outcomes for brain arteriovenous malformations in children: a systematic review and meta-analysis. Am J Roentgenol. 2020 Aug;215(2):472–87.
36. Richard SA, Shrestha SS, Zhang C, Fu W, Wang T, Cong W, et al. Successful treatment of a child with ruptured arteriovenous malformation using onyx embolization: a case report. Open J Mod Neurosurg. 2017 Oct;7(4):153–63.
37. Sappenfield EC, Jha RT, Agazzi S, Ros S. Cerebral arteriovenous malformation rupture in pregnancy. BMJ Case Rep. 2019 Jul 23;12(7):e225811.
38. Nuñez M, Quintana V, Pereira S. [ Cesarean section in a patient with a large cerebral arteriovenous malformation: anesthetic considerations]. Anest Analg Reanim. 201225(1):39–42. Spanish.
Patricio García-Espinosa, 1 Medical Student. School of Medicine, Universidad Autónoma de Nuevo León, Monterrey. México
Edgar Botello-Hernández, 1 Medical Student. School of Medicine, Universidad Autónoma de Nuevo León, Monterrey. México
Gabriela Torres-Hernández, 1 Medical Student. School of Medicine, Universidad Autónoma de Nuevo León, Monterrey. México
Clarissa Guerrero-Cavazos, 1 Medical Student. School of Medicine, Universidad Autónoma de Nuevo León, Monterrey. México
Estefania Villareal-Garza, 2 MD. Neurology Service, “University Hospital “José Eleuterio González”, Universidad Autónoma de Nuevo Leon, Monterrey. México
Andrea Flores-Rodriguez, 1 Medical Student. School of Medicine, Universidad Autónoma de Nuevo León, Monterrey. México
About the Author: García-Espinosa, P. is currently a sixth-year medical student of UANL medical school, Monterrey, Mexico of a six-year program. He is also current leader of GECEN researchers (Group of students against neurological diseases, undergraduate neurology department arm).
About the Author: Botello-Hernández, E. is currently a fifth-year medical student of UANL medical school, Monterrey, Mexico of a six-year program. He is also member of GECEN researchers.
About the Author: Torres-Hernández, G. is currently a sixth-year medical student of UANL medical school, Monterrey, Mexico of a six-year program. She is also member of GENEP investigators (Group of students focused on pediatric neurology).
About the Author: Guerrero-Cavazos, C. is currently a fifth-year medical student of UANL medical school, Monterrey, Mexico of a six-year program. She is also member of GECEN researchers.
About the Author: Villareal, Garza E. is currently a pediatric neurology second year resident at UANL medical school, Monterrey, Mexico.
About the Author: Flores-Rodríguez, A. is currently a sixth-year medical student of UANL medical school, Monterrey, Mexico of a six-year program. She is also member of KER unit Mexico.
Correspondence: Patricio García-Espinosa. Address: Pedro de Alba S/N, Niños Héroes, Ciudad Universitaria, NL, Mexico. Email: patricio.garciaes@uanl.edu.mx
Editor: Nate Hayward & Ciara Egan Student Editor: Adnan Mujanovic Copyeditor: Adnan Mujanovic & Purva Shah Proofreader: Ciara Egan Layout Editor: Ana M. Morales Process: Peer-reviewed
Cite as: Garcia-Espinosa P, Botello-Hernández E, Torres-Hernández G, Guerrero-Cavazos C, Villareal-Garza E, Flores-Rodriguez A. Predictors of Cerebral Arteriovenous Malformation Mortality: A Single-center, Five-year Retrospective Study. Int J Med Students. 2021 Jul-Sep;9(3):213-8.
Copyright © 2021 Patricio García-Espinosa, Edgar Botello-Hernández, Gabriela Torres-Hernández, Clarissa Guerrero-Cavazos, Estefania Villareal-Garza, Andrea Flores-Rodriguez
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 9, NUMBER 3, September 2021