Case Report
Splenic Rupture in a COVID-19 Patient – A Case Report
Anna C. Crowley1, Raul R. Magadia2, Arianna B. Lanpher3
doi: http://dx.doi.org/10.5195/ijms.2021.912
Volume 9, Number 3: 219-222
Received 15 01 2021:
Rev-request 04 03 2021:
Rev-request 23 04 2021:
Rev-request 02 06 2021:
Rev-recd 30 03 2021:
Rev-recd 12 05 2021:
Rev-recd 09 07 2021:
Accepted 10 07 2021:
Publication 16 07 2021
ABSTRACT
Abstract
Background:
It is well known that the Coronavirus disease 2019 (COVID-19) causes coagulation changes,
requiring frequent monitoring for potential sequelae such as myocardial infarction
and stroke. Non-traumatic splenic rupture is a rare and poorly understood occurrence
in the clinical setting. Possible causes of nontraumatic splenic rupture include neoplasm,
infection, inflammatory disease, iatrogenic and mechanical causes. Furthermore, increased
intrasplenic tension, increased abdominal pressure, and thrombotic vascular occlusion
are possible mechanisms.
The Case:
We report a case of splenic rupture in a COVID-19 patient. Our patient was a 52-year-old
black man, presenting with diarrhea and moderate dyspnea, who was found to be COVID-19
positive. He had a past medical history significant for end-stage renal disease, chronic
anemia, and aortic valve replacement. In an otherwise uneventful, 7-day hospital course,
the patient's stay abruptly resulted in a nontraumatic splenic rupture and demise.
In this report, we have evaluated the likelihood of COVID-19 causing splenic rupture
in a patient with no prior splenic disease.
Conclusion:
This case highlights the possibility of splenic rupture in otherwise normally recovering
COVID-19 patients, particularly in the presence of comorbid conditions of renal failure
and anticoagulation, with increased abdominal pressure during routine defecation.
This information may assist in furthering the pathophysiology of COVID-19 and its
life-threatening complications. In patients with COVID-19, non-traumatic splenic rupture
should be considered as one of the differential diagnoses in patients who present
with abdominal pain and early recognition of the same, owing to a high index of suspicion,
can be lifesaving.
Keywords:
Case report;
COVID-19;
Splenic rupture (Source: MeSH-NLM)..
Introduction
While the Coronavirus disease 2019 (COVID-19) is known to present with significant
pulmonary and cardiac manifestations, other systemic complications and interactions
with pre-existing pathology are being recognized.1 As the pandemic has evolved, hypercoagulability and microvascular changes are becoming
more prevalent causes of mortality.2 Here, we describe a case of nontraumatic splenic rupture in a COVID-19 patient being
treated with anticoagulants and routine hemodialysis. Atraumatic splenic rupture is
exceedingly rare and a potentially fatal condition. Causes of atraumatic splenic rupture
include neoplasms, infection, iatrogenic, mechanical and inflammatory states.3 This case illustrates how interactions with chronic renal disease and anticoagulation
use may be important considerations in the treatment of complicated COVID-19 patients.
The Case
History of Present Illness
A 52-year-old Black male presented to the Emergency Department with a chief complaint
of diarrhea for 1 day, followed by moderate dyspnea. At the time of admission, the
BUN/Cr ratio was 44/11.3, and on admission, the SARS-CoV-2 antigen by IFA (in-house,
Sofia) was positive. The patient was afebrile (36.9 °C), and the oxygen saturation
was 100% on room air.
Past Medical History
He had a past medical history of hypertension, hepatitis B (2006), end-stage renal
disease (2005), hyperlipidemia, severe anemia (2008), and aortic valve replacement
(2019). Current medications included warfarin (10mg daily, in addition to supplemental
5mg on M/W/F) due to past mechanical aortic valve replacement. The patient's goal
INR was 2.5–3.5. The patient had a history of hemodialysis noncompliance and often
did not attend the recommended treatments. From laboratory data in 2019, the patient's
average BUN/Cr was 34.2/6.5, respectively. Patient denied any recent travel. He did
not have any personal or family history of leukemia, lymphoma, coagulopathies, DVT/PE,
auto-immune pathology, or other neoplasms.
Highlights:
- Presentation of a unique case of COVID-19 complicated by non-traumatic splenic rupture.
- Diagnostic dilemma of conflicting coagulation studies in a COVID-19 patient with chronic
renal failure requiring hemodialysis and valve replacement requiring warfarin therapy,
leading to splenic rupture, a complication that is associated with hypocoagulable
state.
- Highlights possibility of fatal splenic rupture in COVID-19 patients with comorbid
renal disease and complex coagulation states, reminding clinicians that rapid diagnosis
and surgical correction can be life-saving.
Investigations
Laboratory results on admission were significant for pancytopenia, elevated inflammatory
markers, and elevated coagulation studies. From laboratory data in 2019, the patient
had a history of leukopenia, anemia, platelet count of 160.7 (103/μL), and an average Hgb/Hct of 8.2/25.6 (g/dL/%), respectively. Physical exam revealed
normal lung sounds, no hepatosplenomegaly, or lymphadenopathy. Prior to this current
admission, the patient was evaluated for further renal disease progression in December
2018. There was no evidence of splenic injury or splenomegaly on the 2018 abdominal
CT. The patient's pancytopenia was evaluated during his stay at the hospital; both
nutritional and viral etiologies, as well as bone marrow failure, were ruled out with
appropriate investigations, as shown in Table 1. Peripheral smear was unremarkable.
Table 1.
Inpatient laboratory values.
| Test |
Ref. Range |
Day 0 |
D1 |
D2 |
D3 |
D4 |
D5 |
D6 |
D7 |
| WBC (103/μL) |
4.5–10.4 |
1.5 |
1.6 |
1.7 |
1.8 |
2.8 |
3.5 |
5.2 |
5.4 |
| RBC (106/μL) |
3.7–5.3 |
2.41 |
2.37 |
2.11 |
2.29 |
2.10 |
1.64 |
2.04 |
1.91 |
| Hgb(g/dL)/HCT (%) |
11.0–16.0/35.0–47.0 |
7.8/23.5 |
7.4/22.9 |
6.6/20.8 |
6.9/22.2 |
6.5/20.4 |
5.2/16.5 |
6.5/19.9 |
6.0/18.2 |
| Platelet (103/L) |
140–440 |
54 |
62 |
60 |
59 |
76 |
68 |
72 |
69 |
| INR |
|
2.93 |
– |
2.24 |
2.22 |
2.84 |
5.13 |
4.65 |
4.73 |
| PT (s) |
9.8–11.6 |
29.8 |
– |
23.1 |
22.9 |
28.9 |
50.8 |
46.2 |
47 |
| aPTT (s) |
23.1–31.6 |
56.6 |
– |
|
|
|
|
|
|
| AST (unit/L) |
2–33 |
24 |
|
|
|
|
|
|
|
| ALT (unit/L) |
13–61 |
12 |
|
|
|
|
|
|
|
| Albumin (gm/dL) |
3.4–5 |
2.7 |
|
|
|
|
|
|
|
| Ferritin (ng/mL) |
8.0–252.0 |
2184.6 |
– |
|
|
|
|
|
|
| CRP (mg/L) |
0.0–3.0 |
24.5 |
– |
|
|
|
|
|
|
| Procalcitonin (ng/mL) |
<0.10 |
0.52 |
– |
|
|
|
|
|
|
| Sed Rate (mm/h) |
0–30 |
53 |
– |
|
|
|
|
|
|
| D-dimer |
0.19–0.5 |
2.92 |
– |
|
|
|
|
|
|
| LDH (unit/L) |
87–241 |
360 |
– |
|
|
|
|
|
|
| BUN/Cr |
7–18/0.6–1.3 |
44/11.3 |
– |
|
|
|
|
|
|
| Lymphocyte (%) |
|
25.5 |
43.0 |
36.5 |
32.6 |
27.3 |
25.1 |
22.9 |
16.5 |
| Mon ocyte (%) |
|
9.4 |
13.3 |
17.1 |
11.4 |
9.1 |
13.6 |
12.9 |
11.9 |
Hospital Course: On the second day of admission, the patient received dialysis, and
over the next six days underwent a total of three hemodialysis treatments coupled
with three separate packed RBC transfusions because of his severe anemia. His chest
radiograph remained clear and diarrhea subsided by Day 2. Due to the belief that the
patient's symptoms were due to COVID-19 initially, no abdominal imaging was performed
on admission. On Day 5, the INR increased to 5.13 and remained above the therapeutic
range (2.5–3.5) for the remainder of his hospital course. After the patient's increase
in coagulation studies, his regular warfarin treatment regimen (10mg daily, in addition
to supplemental 5mg on M/W/F) was discontinued on day 6.
On Day 8, when attempting defecation, the patient felt a “pop” and developed tearing
and burning LLQ flank pain at 8:00. This bowel movement was blood-tinged and loose.
His abdominal pain progressed throughout the day, and at 16:00, the internist's physical
examination revealed a firm abdomen with no rebound tenderness. CT scan of the abdomen
was compatible with acute splenic hemorrhage. Before emergency splenectomy could be
performed, the patient became hypotensive, developed cardiac arrest at 18:55, and
expired at 19:12.
The abdominal CT detected a large amount of heterogenous material surrounding the
spleen with small to moderate amount of free fluid around the spleen, compatible with
acute splenic hemorrhage, as shown in Figure 1. Due to the abrupt onset of acute intra-abdominal hemorrhage, the patient died prior
to splenectomy or other life-saving interventions. No autopsy was performed.
Figure 1.
Abdominal CT confirming splenic rupture
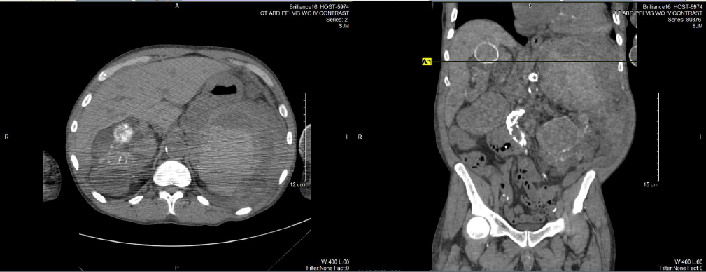
Legend: Impression Per Radiology: 1. Large amount of heterogenous material surrounding the
spleen with small to moderate amount of free fluid in the abdomen compatible with
acute splenic hemorrhage. 2. Renal findings compatible with autosomal dominant polycystic
kidney disease. 3. Small bilateral pleural effusions, greater on the left. 4. Atherosclerosis
Throughout the patient's stay, he received the standard in-house COVID-19 treatment,
including five days of Ascorbic acid (50 mL 2000mg/4 mL IV), Thiamine HCl (2mL 200
mg/2 mL IV), and Zinc Sulfate (20 mg oral QD), along with his continued warfarin anticoagulation
as previously prescribed until its discontinuation on Day 6.
Discussion
We believe this case of non-traumatic splenic rupture in a COVID-19 patient was caused
by interactions from COVID-19-related coagulation changes. Based on recent data, COVID-19
has been shown to cause both hypercoagulable and hyperfibrinolyic states in patients.2 Because our patient was on chronic warfarin therapy and reached supratherapeutic
levels during hospital days 5-8, we believe the effects of COVID-19 affected our patient's
coagulability and possibly led to his hemorrhagic state and splenic rupture.
While the exact etiology of nontraumatic splenic rupture is not fully understood,
three possible mechanisms could explain this patient's unfortunate clinical course:
increased intrasplenic tension, increased abdominal pressure, and altered coagulation.4 We believe the supratherapeutic warfarin levels and hyperfibrinolytic state caused
by COVID-19 led to our patient's altered coagulation studies. The process of defecation
is a known inciting event for splenic rupture due to the rising intrabdominal pressure
and stretching of the splenocolic ligament causing rupture of pre-existing subcapsular
hematoma. While the patient had no evidence of pre-existing splenic hematoma or splenomegaly,
we believe this was the chief inciting event leading to splenic rupture.5
It is relevant to eliminate the other causes of nontraumatic splenic rupture. Because
our patient did not receive an autopsy following his death, we cannot be certain that
our patient did not have an underlying primary splenic neoplasm (i.e., splenic marginal
zone lymphoma) or primary myelofibrosis. However, these etiologies are extraordinarily
rare.6 Our patient did not present with any clinical or laboratory findings suggestive of
an underlying hematological malignancy, and there was no hepatosplenomegaly, enlarged
lymph nodes, or systemic B symptoms. Mature B- or T-cell leukemias are unlikely because
the patient's lymphocyte count was within the normal range. Hairy cell leukemia can
be ruled out because peripheral blood smear typically reveals a pancytopenia with
monocytopenia. Primary myelofibrosis typically presents with an enlarged spleen and
liver with tear drop cells on blood smear, which was not detected in our patient.
Epstein Barr Virus and cytomegalovirus were not suspected due to the absence of atypical
lymphocytes and leukocytosis.
Even though microvascular changes, coagulation changes, and defecation can be regarded
as the principal causes of splenic rupture in this case, the consequences of repetitive
hemodialysis from chronic renal disease cannot be overlooked. This is a rare reported
complication of hemodialysis, and its exact incidence is not known. However, in a
previous study of nontraumatic splenic rupture in a hemodialyzed patient, important
risk factors included the use of anticoagulants during hemodialysis, uremic coagulopathy,
susceptibility to infections, and impaired immune function. These risk factors can
occur as long-term complications of hemodialysis, but they are also complications
of severe coronavirus infection, which paradoxically is associated with coagulation
changes.7 The exact etiology and pathogenesis cannot be confirmed due to the lack of an autopsy,
and because of the extremely low incidence of splenic rupture due to hemodialysis,
and the absence of known risk factors in our patient (e.g., infectious mononucleosis,
hematologic disease, splenomegaly, neoplasm). Therefore, we believe COVID-19 infection
was a contributing cause of splenic rupture in our patient.
There have been other recently reported cases of nontraumatic splenic rupture in the
setting of COVID-19. Research demonstrates that COVID-19 has a direct effect on the
body's secondary lymph tissue. Following these studies, there is further reason to
suspect the virus has the potential to have a direct effect on the spleen by causing
“lymphoid follicle attrition and nodular atrophy in addition to microvascular thrombosis
and necrosis,” as stated in a case report by Shaukat and colleagues.8
In monitoring COVID-19 progression, clinicians monitor inflammatory markers, such
as C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, and lymphocytes.
As shown in Table 1, several other laboratory values are now closely monitored to evaluate the coagulation
changes related to the COVID-19 pathogenesis including d-dimer, fibrinogen, prothrombin
time, partial thromboplastin time, platelet count, and other specific quantifications
such as calcium.9 Although the mechanism is still unknown, elevated coagulation markers support studies
documenting many critically ill COVID-19 patients who suffer from a thrombotic microvascular
event.10 Studies have suggested that in the setting of COVID-19, symptoms such as abdominal
pain may be an indication for abdominal CT scan on admission and frequent monitoring
throughout the patient's disease progression. In a reported case of splenic rupture
in Poursina Hospital in Rasht, Iran, a COVID-19 patient had vague abdominal symptoms
and subsequent signs of decompensation. Urgent laparotomy was performed, revealed
atraumatic splenic rupture, and splenectomy was performed. Fortunately, the acuity
of these physicians’ actions were able to save the patient's life.11
Due to the multisystem involvement of COVID-19, coagulation studies are becoming increasingly
relevant in that the virus can cause both hypercoagulable and hemorrhagic changes.
We are assuming that our patient's hyperfibrinolytic state led to his splenic hemorrhage.
While the coronavirus remains a heavily studied topic both microbiologically and clinically,
it is pertinent that clinicians grow more cognizant of emerging complications related
to COVID-19. This case highlights the importance of monitoring coagulation studies
while maintaining a high index of suspicion for rare but life-threatening intra-abdominal
complications. In patients with COVID-19, non-traumatic splenic rupture should be
considered as one of the differential diagnoses in patients who present with abdominal
pain and early recognition of the same, owing to a high index of suspicion, can be
lifesaving.
Acknowledgments
None
Conflict of Interest Statement & Funding
The Authors have no funding, financial relationships or conflicts of interest to disclose.
Author Contributions
Conceptualization: CAC, RRM, ABL. Visualization: CAC, ABL. Data Curation, Investigation,
Project Administration, Writing – Original Draft Preparation: CAC. Supervision, Validation:
RRM, Writing – Review and Editing: RRM, ABL.
References
1. Temgoua MN, Endomba FT, Nkeck JR, Kenfack GU, Tochie JN, Essouma M. Coronavirus Disease 2019 (COVID-19) as a Multi-Systemic Disease and its Impact in
Low- and Middle-Income Countries (LMICs). SN Compr Clin Med. 20201–11.
2. Kelsey Gockman, Alyssa Harbaugh, Yogendra Kanthi, Jason S. Knight, Daniel A. Lawrence, Jacqueline A. Madison, et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized
COVID-19 patients. Scientific Reports. 2021 Jan;11:1580.
3. D. Candinas, B. Gloor, A Hostettler, P. Renzulli, A. M. Schoepfer. Systematic Review of Atraumatic Splenic Rupture. Br J Surg. 2009 Oct;96(10):1114–21. doi: http://dx.doi.org/10.1002/bjs.6737
 .
.
4. Ayfer Aktas, Mustafa Aldemir, Ercan Gedik, Sadullah Girgin, Mustafa Aldemir, Celalettin Keles, Mehmet Cudi Tuncer. Non-Traumatic Splenic Rupture: Report of Seven Cases and Review of the Literature. World Journal of Gastroenterology. 2008 Nov; 14(43):6711–6.
5. Chikashi Gotoh, Kouji Masumoto, Kentara Ono, Toko Shinkai, Yasuhisa Urita. A Rare Mechanism of Delayed Splenic Rupture Following the Nonoperative Management
of Blunt Splenic Injury in a Child. Surgical Case Reports. 2018 Dec; 4:75.
6. M. M. Al-Hawary, S. Azar, R. K. Kaza, I.R. Francis. Primary and Seconday Neoplasms of the Spleen. 2010 Aug; 10(1):173–82.
7. Se-Ho Chang, Hyun-Jung Kim, Gyeong-Won Lee, Jong Deog Lee, Dong Jun Park. Spontaneous Splenic Rupture in a Hemodialysis Patient. Yonsei Medical Journal, Yonsei University College of Medicine. 2005 Jun 30; 46(3):435–8.
8. Shaukat I, Khan R, Diwakar L, Kemp T, Bodasing N. Atraumatic splenic rupture due to covid-19 infection. Clinical infection in practice. 2021 Apr 1;10:100042.
9. Ronghui Du, Guohui Fan, Ying Liu, Zhibo Liu, Ting Yu, Fei Zhou, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in
Wuhan, China: a Retrospective Cohort Study. The Lancet. 2020 Mar; 395(10229):1054–62.
10. David Berlin, Joanna Harp, Jeffrey Laurence, Cynthia Magro, J. Justin Mulvey, Steven Salvatore, et al. Complement Associated Microvascular Injury and Thrombosis in the Pathogenesis of Severe
COVID-19 Infection: A Report of Five Cases. Translational Research: The Journal of Laboratory and Clinical Medicine. 2020 Jun; 220: 1–13.
11. Mobayen, M., Yousefi, S., Mousavi, M., & Anbaran, A. S. (2020). The presentation of spontaneous splenic rupture in a COVID-19 patient: a case report. BMC surgery, 20(1):1–5.
12. Judith A. James, Doruk Erkan, Joan T. Merrill, Jerald Winakur. Emerging Evidence of a COVID-19 Thrombotic Syndrome Has Treatment Implications. Nature Reviews Rheumatology. 2020 July; 16:581–9.
Anna C. Crowley, 1 BS, Alabama College of Osteopathic Medicine, Dothan, AL 36303, United States.
Raul R. Magadia, 2 MD, Northeast Alabama Regional Medical Center, Infectious Disease, United States.
Arianna B. Lanpher, 3 BA, Alabama College of Osteopathic Medicine, Dothan, AL 36303, United States.
About the Author: Anna Crowley is currently a 3rd year medical student of the Alabama College of Osteopathic
Medicine, Dothan, Alabama, of a 4-year program. She holds a B.S. degree in Chemical
Engineering from Auburn University, and while in her clinical years at ACOM, she has
become passionate about preventative healthcare and furthering patient education.
She is the recipient of the Council of Osteopathic Student Presidents Association
Gold Badge Volunteer Award.
Correspondence: Anna C. Crowley. Address: Alabama College of Osteopathic Medicine, Dothan, AL 36303,
USA. Email: crowleya@acom.edu
Editor: Francisco J. Bonilla-Escobar
Student Editors: Rahul Abraham & Najdat Bazarbashi
Copyeditor: Madeleine Jemima Cox
Proofreader: Adam Dinnof
Layout Editor: Sushil Dahal
Process: Peer-reviewed
Cite as: Crowley, AC, Magadia, RR, Lanpher AB. Splenic Rupture in a COVID-19 Patient – Case
Report. Int J Med Students. 2021 Jul-Sep;9(3):219-22.
Copyright © 2021 Anna C. Crowley, Raul R. Magadia, Arianna B. Lanpher
This work is licensed under a Creative Commons Attribution 4.0 International License.
International Journal of Medical Students, VOLUME 9, NUMBER 3, September 2021

