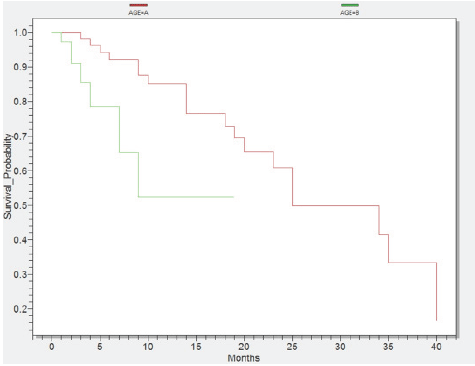
Survival probability according to age Group A1 <60years (red) and Group B1 ≥60years (green)
Alina Maria Gridjac1, Cristian Daniel Pirlog2, Anca Simona Bojan3
doi: http://dx.doi.org/10.5195/ijms.2013.207
Volume 1, Number 2: 70-73
Received 30 04 2012: Accepted 02 03 2013
ABSTRACT
Background:Acute myeloid leukemia (AML) is a malignant disease with significant identified prognostic factors. Therefore our aim was to develop an Assessment Scheme of Prognosis in AML based on prognostic factors. In some counties, such as Romania or other less-highly developed countries, this scheme would be beneficial particularly when cytogenetic testing is unavailable or time-intensive.
Methods:We analyzed 119 adult patients with AML during a five year-period from a single-center in Romania. We retrospectively collected and analyzed data with Epi Info and Excel using patient medical records.
Results:According to age, the group A1 (<60 years) had a 40 months survival, in contrast with the group B1 (≥60 years) with a survival of 19 months (p=0,0063). The group A2 (secondary AML) survived 15 months, whereas the group B2 (AML de novo) survived 40 months (p=0.0021). Additionally, the group A3 (mild comorbidities) achieved a 40 months survival, the group B3 (moderate comorbidities) survived 19 months, whereas the group C3 (severe comorbidities) survived 7 months (p=0,0059). According to WBC and blast number, the group A4 (high levels) had a 25 months survival, whereas the group B4 (low levels) survived 40 months (p=0,0057).
Conclusion:The prognostic factors studied are useful to identify the risk level of AML disease for each patient at diagnosis. We developed an assessment scheme of prognosis with three risk groups according to age, secondary AML, comorbidity, WBC and blasts and cytogenetic examination.
Keywords: Acute Myeloid Leukemia; Survival Analysis; Prognosis; Risk Assessment; Age Factor.
Acute myeloid leukemia (AML) is a clonal disease characterized by the proliferation and accumulation of myeloid progenitor cells in bone marrow, which ultimately leads to hematopoietic failure.1,2 The incidence of AML increases with age, and older patients typically have worse treatment outcomes than younger patients.3 Diagnosis of AML is based on cellular morphology, immunology, cytogenetics, and molecular features.4
A number of prognostic factors have been identified in AML, including age, performance status, organ dys-function, secondary AML, white blood cell and blast count at presentation, karyotype, and molecular abnormalities.5,6 Most relevant studies, based on large multicenter trials have definitively demonstrated that age and cytogenetics at diagnosis are the most significant prognostic determinants for patients with AML.7
This study focuses on survival rate, prognostic factors and the evolution of AML in patients. The purpose of this study was to formulate an Assessment Scheme for Prognosis of AML. Even if these prognostic factors were previously studied our goal was to develop a score, which is easy to calculate, for early prognostic assessment at diagnosis. Especially in Romania or other less-highly developed countries, this scheme would be beneficial particularly when cytogenetic testing is unavailable or time-intensive.
We performed a retrospective longitudinal study on 119 patients, aged 21-85 years with a median age of 60. Using statistical tests such as Excel and Epi Info we managed to associate, analyze and compare the variables. Additionally, this study was analytical and observational.
The length of the study was five years, from January 2007 to November 2011. The main feature of all patients was the presence of AML. The demographic characteristics included the patients admitted at the Department of Hematology of the Oncology Institute “Prof. Dr. Ion Chiricuta”, Cluj-Napoca, Romania. Although it was a single-center study, it comprised a whole region of Romania, because Cluj-Napoca is the most important medical centre in Transilvania.
We aimed to identify the most important prognostic factors, therefore to perform a risk assessment for patient with AML. According to Kaplan-Meier’s method, we analysed the patient’s survival rate to illustrate the differences between specific variables and the characteristics of the patients.
We formulated five research hypotheses, which confirmed a shorter survival for 60 year olds, secondary AML patients, patients with severe comorbidities and patients with elevated levels of WBC and blasts.
The data used in the study comprised: gender, age, place of origin, date of admission, FAB type, WHO classification, cytogenetic examination, immunophenotyping, laboratory data (hemoglobin, erythrocytes, leukocytes, platelets, LDH, blasts), symptoms, comorbidity, other malignancies, dysplasia, treatment, complications, date of death and status censorship. Status of censorship coding included two groups of patients. The first group was “censored” if at the end of study, some patients were lost, whereas the second group was “complete” if during the study, some patients died.
Histogram of the age intervals was significantly high among patients over 50 year old (77%). In addition, 34 patients were included in the 50-59 years interval, followed by 31 cases in the sixth decade interval and 27 cases included in the seventh decade until the age of 85 years. In Figure 1 we represented a statistical test conducted in Epi Info, where all patients were divided in two groups: group A1 <60 year olds and group B1 ≥60 year olds. Consequently, the group A1 achieved a survival of 40 months and group B1 a 19 month survival (p=0,0063).
Figure 1.Survival probability according to age Group A1 <60years (red) and Group B1 ≥60years (green)

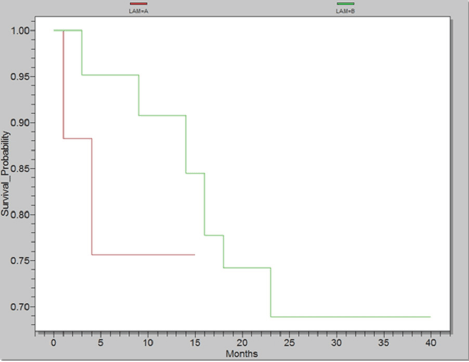
Almost 14% presented with other malignancies requiring radio-chemotherapy. As a result, patients with these malignancies developed secondary AML. Moreover, half of all cancer patients presented with genitourinary malignancy whilst a third were gastrointestinal in origin. Analysis of the survival of primary and secondary AML patients revealed significant differences. As shown in Figure 2, there was a 15 month survival for the first group (A2 =Secondary AML) and 40 month survival for the last group (B2 =AML de novo, p=0,0021).
Figure 2.Survival probability according to presence of secondary AML, Group A2 with Secondary AML (red) and Group B2 with AML de novo (green)
Prognosis assessment scheme.
| Points | Age (years) | Secondary AML | Comorbidity | WBC (cells/mm3) | Blasts (%) | Cytogenetics |
|---|---|---|---|---|---|---|
| 1p | <40 | - | Mild | <15000 | <40% | t (15,17), t (8,21), inv16* |
| 2p | 40-59 | Present | Moderate | 15,000-100,000 | 40-80% | Normal karyotype* |
| 3p | >59 | Present with other malignancies | Severe | >100,000 | >80%* | del (7q), +8, del (9q), t(v,11)(v,23)* |
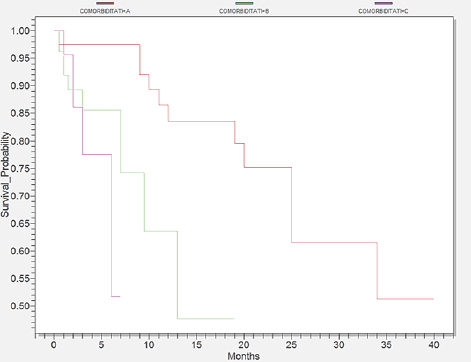
A proportion of 64% of cases had associated comorbidities as they were present especially within the elderly patients. The most frequent were the heart diseases, which comprised 60 cases. Digestive and respiratory illnesses ranked next with 28 and 21 cases respectively. The next frequent disease was diabetes with 19 cases and the less frequent diseases were renal and neurological disorders with 10 and 9 cases respectively. We separated patients, according to comorbidities into three groups seen in Figure 3. Group A3 comprised patients with mild comorbidity with a survival of 40 months. Group B3 covered patients with moderate comorbidities who survived 19 months, whereas group C3 included patients with serious comorbidities who survived only 7 months (p=0,0059).
Figure 3.Survival probability according to comorbidity, Group A3 with mild comorbidity (red), Group B3 with moderate comorbidity (green) and Group C3 with severe comorbidity (pink)
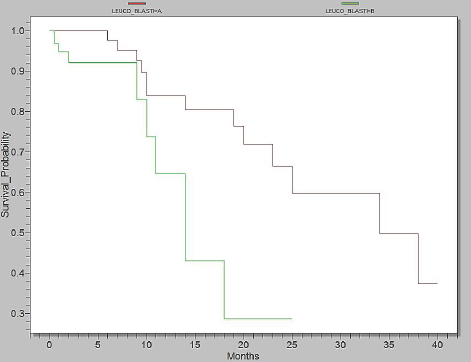
As shown in the Figure 4, in Epi Info, we analyzed two categories: group A4 =WBC <15.000/mm3 + blasts <40% and group B4 =WBC >15.000/mm3 + blasts >40%. Survival in group A4 was up to 40 months, whereas the group B4 had a survival of 25 months (p=0,0057).
Figure 4.Survival probability according to level of WBC and Blasts, Group A4= WBC <15.000/mm3 + blasts <40% (red) and Group B4= WBC >15.000/mm3 + blasts >40% (green)
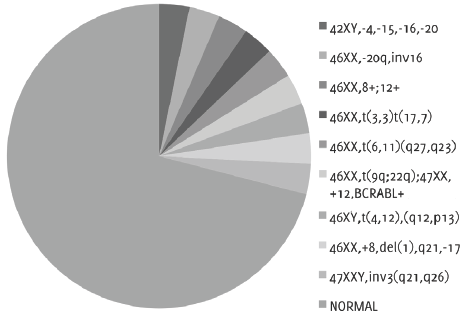
Only 26% of patients received cytogenetic examination. The prognosis distribution of results followed the WHO classification, therefore 71% of patients correspond to intermediate risk, whereas 29% of patients correspond to unfavorable risk with chromosomal abnormalities (Figure 5).
Figure 5.Cytogenetic examination with presence of chromosomal abnormalities or normal karyotype
Various clinical and biological features identified previously as useful factors in the prediction of clinical outcomes, could help guide therapy choices.8 Large multicenter trials have demonstrated that: patient’s age, presence of secondary AML, WBC count, cytogenetic and comorbidities at diagnosis were important prognostic factors for AML patients.9
Age remained one of the most significant prognostic factors and its importance has increased among the elderly population.10 Our study shows a clear difference of survival period between the two age groups: <60, >60 years. We decided to define a 60 year-limit because this age is the limit between adults and elderly people. We were not able to find the sample size variable due to the small number of patients. We recognized age as a vital prognostic factor in AML, so we included it in our prognosis scheme.
We highlighted the importance of prior malignancies and their treatment that had a major impact on our patients survival rate.11 Furthermore, patients with previous malignancies developed secondary AML, which in our study illustrated a surprisingly low life expectancy. As a result, patients with AML de novo lived twice as long as the patients with secondary AML.
Comorbidities affect the therapeutic plan and the post-therapeutic outcome of the index disease. Examples include patients with cancer and multiple studies have demonstrated the relevance of comorbidities in prognosis.12,13 Consequently, we stratified comorbidities after the Sorror Comorbidity Index in mild, moderate and severe comorbidities. Kaplan-Meier survival illustrated a significant difference between the groups in our study. As a result, comorbidities are an important prognostic factor, which affects the survival of patients.
Besides cytogenetic and molecular abnormalities, classically, a high WBC count at presentation was considered an independent prognostic factor. This illustrated a poor outcome in AML, especially for the cytogenetic intermediate risk group.13 A subgroup of patients with hyperleucocytosis was identified with a shorter rate of survival in these cases.14 In our study, many laboratory analyses were performed, but we chose to combine WBC and blast count for a significant difference on our patients survival. Patients were divided into groups according to low or high levels of WBC and blasts. Patients with values of WBC and blasts >40% had much shorter survival than those with low values.
Karyotype abnormalities are probably the most important prognostic determinants in AML.15 However, in the literature it is reported that half of adult AML patients present with a normal karyotype.16 In our study, a proportion of 71 per cent presented a normal karyotype and 29 per cent had unfavorable cytogenetic abnormalities. In our study, we were unable to demonstrate the significance of cytogenetic testing due to the small number of patients who benefited from cytogenetic examination. Finally, we propose that all AML patients should receive cytogenetic examination for a better management of AML.
Our single-center study evaluated and confirmed the significant predictors of outcome of AML. The age of patients, the presence of secondary AML, comorbidities and WBC count and blasts were the most important prognostic parameters. Furthermore, we demonstrated this in our study with the support of significant results of survival rates among the different groups of patients.
The understanding of dominant prognostic factors in patients with AML is rapidly evolving, but until we find more accurate factors, we should clarify the known ones. This single-center study evaluated the significance of pretreatment factors, and found that the age of patients, the presence of secondary AML, comorbidities, WBC, blasts and cytogenetics were factors which influenced the outcomes of survival in patients with AML. Therefore, we propose an Assessment Scheme of Prognosis for patients with AML, which would be useful to apply a therapeutic regimen and to categorize patients into risk groups. Furthermore, we can assign patients with AML according to their prognosis, in three categories: Low risk, intermediate risk and high risk. We included cytogenetic examination with data from medical literature, as we were not able to come up with our own results, due to our small number of patients who received cytogenetic examination. Moreover, we are going to check this scheme on a larger patient population to demonstrate its significance. On some patients we have already evaluated this scheme and we obtained a precise prognosis. The scheme works by adding the points if the factor is present. For instance, a patient who is aged ≥60 years (3 points), has AML de novo (1 point), moderate comorbidities (2 points), WBC=15000-100.000/mm3 and blasts=40-80% (2 points) and an unfavorable chromosomal abnormality (3 points) would be classified as high risk. Finally, this classification would also help direct the precise therapy for this patient.
None.
The Authors have no funding, financial relationships or conflicts of interest to disclose.
1. Kaushansky K, Lichtman MA, Beutler E, et al. Williams Hematology 8-th edition. New York, The McGraw Hill Companies Editure, 2010.
2. Liesveld JL, Lichtman MA. Acute Myelogenous Leukemia. In: Williams Hematology, 8-th edition, New York, The McGraw Hill Companies Editure, 2010, pp.1277–1312.
3. Schneider F, Hoster E, Schneider S, Dufour A, Benthaus T, Kakadia PM, et al. Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML). Ann Hematol. 2012;91(1):9–18.
4. Hartmut D, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia. Blood. 2010;115(3):453–74.
5. Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica 2010;95(11)1857–64.
6. Wagner K, Damm F, Thol F, et al. FLT3-internal tandem duplication and age are the major prognostic factors in patients with relapsed acute myeloid leukemia with normal karyotype. Haematologica 2011;96(5):681–6.
7. Schellongowski P, Staudinger T, Kundi M, et al. Prognostic factors for intensive care unit admission, intensive care outcome, and post-intensive care survival in patients with de novo acute myeloid leukemia: a single center experience. Haematologica 2011;96(2):231–7.
8. Petrie K, Zelent A, Waxman S. Differentiation therapy of acute myeloid leukemia: past, present and future. Curr Opin Hematol 2009;16(2):84–91.
9. Colovic N, Tomin D, Vidovic A, et al. Pretreatment prognostic factors for overall survival in primary resistant acute myeloid leukemia. Lancet Oncol 2011;11(6):543–52.
10. Pulte D, Gondos A, et al. Expected long-term survival of patients diagnosed with acute myeloblastic leukemia during 2006–2010. Annals of Oncology 2010;21(2):335–41.
11. Larson R, et al. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia?. Best Pract Res Clin Haematol. 2007;20(1):29–37.
12. Breccia M, Frustaci AM, Cannella L. Comorbidities and FLT3-ITD Abnormalities as Independent Prognostic Indicators of Survival in Elderly Acute Myeloid Leukaemia Patients. Hematol Oncol 2009;27(3):148–53.
13. Sorror ML, Storb R, et al. Role of Comorbidities in Optimizing Decision-Making for Allogeneic Hematopoietic Cell Transplantation. Mediterr J Hematol Infect Dis 2010;2(2):e2010015.
14. Marbello L, Ricci F, Nosari A, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia:A single-center retrospective study and review of literature. Leuk Res. 2008;32(8):1221–7.
15. Gulley M, Shea T, Fedoriw Y, et al. Genetic Tests To Evaluate Prognosis and Predict Therapeutic Response in Acute Myeloid Leukemia. J Mol Diagn 2010;12(1):3–16.
16. Mrozek K, Marcucci G, Pascha P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification?. Blood 2007;109(2):431–48.
Alina Maria Gridjac, 1 Faculty of General Medicine, University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania
Cristian Daniel Pirlog, 2 Faculty of Dental Medicine, University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania
Anca Simona Bojan, 2 Faculty of Dental Medicine, University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania
About the Author: Alina Gridjac is a 6th year medical student at Faculty of General Medicine, University of Medicine and Pharmacy “Iuliu Hatieganu”, Cluj-Napoca, Romania
Correspondence Alina Maria Gridjac, Address: 25 Main Street, Rona de Sus, Maramures, Romania, 437250. Email: alina_gridjac@yahoo.com
Cite as: Gridjac AM, Pirlog CD, Bojan AS. Survival and Prognostic Factors in Adults Acute Myeloid Leukemia: A Retrospective Analysis of 119 Cases. Int J Med Students 2013;1(2):70-3.
Copyright © 2013 Alina Maria Gridjac, Cristian Daniel Pirlog, Anca Simona Bojan
International Journal of Medical Students, VOLUME 1, NUMBER 2, August 2013