Stereological Estimation and Zonal Distribution of the Hepatotoxic Effects of Doxorubicin on the Female Albino Rat (Rattus Norvegicus)
DOI:
https://doi.org/10.5195/ijms.2023.1859Keywords:
Doxorubicin, Hepatotoxicity, Liver, Stereology, Basic science, Liver disease, Pharmacology, Oncology, Cancer treatment, Animal experiment, Experiment, Albino ratAbstract
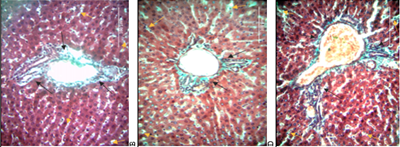
Background: Doxorubicin is an anti-neoplastic agent widely indicated for a variety of cancers. One of its adverse effects is hepatotoxicity which presents with hepatocyte necrosis, sinusoidal dilation, and fibrosis. However, there remains a dearth in the quantification and zonal distribution of this damage.
Methods: Twenty-three adult female Wister albino rats were placed into baseline, control, and experimental group receiving 2.5mg/kg bodyweight Doxorubicin intra-peritoneally thrice weekly for 3-weeks. Rats were sacrificed on days 0, 7, 14 and 21 and livers harvested for processing. Masson’s Trichrome was used in staining 7 µm thick sections. Images were taken and analyzed via STEPanizer, and data entered into SPSS for analysis.
Results: Rats treated with Doxorubicin had increased liver to body weight ratios from 5.00% at baseline to 6.15%, 6.69% and 7.56% on days 7, 14 and 21 (p=0.090). There was a decrease in hepatocyte densities from 51.88/mm2 to 48.61/mm2, 46.65/mm2 and 42.24/mm2 on day 7, 14 and 21 (p=0.779). Collagen fiber deposition increased from 0.12±0.06 cm3 to 0.47±0.55 cm3, 1.64±0.11 cm3 and 1.88±0.24 cm3 on days 7, 14 and 21 (p=0.009). Deposition was greatest periportally and least pericentrally. Volume of sinusoidal spaces increased from 5.46±0.50 cm3 to 5.49±0.15 cm3, 5.53±0.24 cm3 and 5.50±0.17 cm3 on days 7, 14 and 21 respectively (p=0.827). Sinusoids were larger pericentrally than periportally.
Conclusion: Doxorubicin administration is associated with an increase in volume density of fibrotic tissue and sinusoidal spaces but decrease in hepatocytes. The quantitative changes presented may facilitate histopathological grading of Doxorubicin-induced hepatotoxicity.
References
Paridaens R, Biganzoli L, Bruning P, Klijn JGM, Gamucci T, Houston S, et al. Paclitaxel Versus Doxorubicin as First-Line Single-Agent Chemotherapy for Metastatic Breast Cancer: A European Organization for Research and Treatment of Cancer Randomized Study With Cross-Over. J Clin Oncol. 2000;18(4):724. DOI: https://doi.org/10.1200/JCO.2000.18.4.724
Johnson-Arbor K, Dubey R. Doxorubicin. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Available from: http://www.ncbi.nlm.nih.gov/books/NBK459232/. Cited 2021 Jun 15.
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21(7):440–6. DOI: https://doi.org/10.1097/FPC.0b013e32833ffb56
Al-Qzazz MM, Al-Sammak MA, Taher MT. Effect of Doxorubicin on the Histological Structure of the Liver in Male Albino Rats = تأثير عقار الدوكسوروبسين على التركيب النسيجي لكبد الجرذان. Jordan Med J. 2013;47(3):220–6. DOI: https://doi.org/10.12816/0025817
Al-Saleem IA, Jumaa HJ, Al-Ani IM, Ismael HK. Morphological Changes in the Liver of Rats (Rattus norvegicus) treated with different Doses of Doxorubicin. 2017;16:9.
Kipanyula MJ, Sife AS. Global Trends in Application of Stereology as a Quantitative Tool in Biomedical Research. BioMed Res Int. 2018;2018:1–9. DOI: https://doi.org/10.1155/2018/1825697
Marcos R, Monteiro RAF, Rocha E. The use of design-based stereology to evaluate volumes and numbers in the liver: a review with practical guidelines: Design-based stereology in hepatology. J Anat. 2012;220(4):303–17. DOI: https://doi.org/10.1111/j.1469-7580.2012.01475.x
Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Ciênc. 2003;75(4):469–86. DOI: https://doi.org/10.1590/S0001-37652003000400006
Marcos R, Bragança B, Fontes-Sousa AP. Image Analysis or Stereology: Which to Choose for Quantifying Fibrosis? J Histochem Cytochem. 2015;63(9):734–6. DOI: https://doi.org/10.1369/0022155415592180
Vertemati M, Minola E, Goffredi M, Sabatella G, Gambacorta M, Vizzotto L. Computerized morphometry of the cirrhotic liver: Comparative analysis in primary biliary cirrhosis, alcoholic cirrhosis, and posthepatitic cirrhosis. Microsc Res Tech. 2004;65(3):113–21. DOI: https://doi.org/10.1002/jemt.20110
Catta-Preta M, Mendonca LS, Fraulob-Aquino J, Aguila MB, Mandarim-de-Lacerda CA. A critical analysis of three quantitative methods of assessment of hepatic steatosis in liver biopsies. Virchows Arch. 2011;459(5):477–85. DOI: https://doi.org/10.1007/s00428-011-1147-1
Vertemati M, Vizzotto L, Moscheni C, Dhillon A, Dhillon A, Quaglia A. A morphometric model to minimize subjectivity in the histological assessment of hepatocellular carcinoma and its precursors in cirrhosis. Microsc Res Tech. 2008;71(8):606–13. DOI: https://doi.org/10.1002/jemt.20595
Dahab GM, Kheriza MM, El-Beltagi HM, Fouda AMM, El-Din OAS. Digital quantification of fibrosis in liver biopsy sections: Description of a new method by Photoshop software. J Gastroenterol Hepatol. 2004;19(1):78–85. DOI: https://doi.org/10.1111/j.1440-1746.2004.03183.x
Vdoviaková K, Vdoviaková K, Petrovová E, Krešáková L, Maloveská M, Teleky J, et al. Importance Rat Liver Morphology and Vasculature in Surgical Research. Med Sci Monit. 2016;22:4716–28. DOI: https://doi.org/10.12659/MSM.899129
Charan J, Biswas T. How to Calculate Sample Size for Different Study Designs in Medical Research? Indian J Psychol Med. 2013;35(2):121–6. DOI: https://doi.org/10.4103/0253-7176.116232
Yi E tong, Liu R xia, Wen Y, Yin C hong. Telmisartan attenuates hepatic fibrosis in bile duct-ligated rats. Acta Pharmacol Sin. 2012 Dec;33(12):1518–24. DOI: https://doi.org/10.1038/aps.2012.115
Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132(8):1918–26. DOI: https://doi.org/10.1002/ijc.27841
Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Kellogg D. Mol Biol Cell. 2012;23(1):1–6. DOI: https://doi.org/10.1091/mbc.e10-04-0335
Salouege I, Ali R, Saïd D, Elkadri N, Kourda N, Lakhal M, et al. Means of evaluation and protection from doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. J Cancer Res Ther. 2014;10(2):274. DOI: https://doi.org/10.4103/0973-1482.136557
Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3(1):485–513. DOI: https://doi.org/10.1002/cphy.c120014
Maor Y, Malnick S. Liver Injury Induced by Anticancer Chemotherapy and Radiation Therapy. Int J Hepatol. 2013;2013:1–8. DOI: https://doi.org/10.1155/2013/815105
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18. DOI: https://doi.org/10.1172/JCI24282
Forbes SJ, Parola M. Liver fibrogenic cells. Best Pract Res Clin Gastroenterol. 2011;25(2):207–17. DOI: https://doi.org/10.1016/j.bpg.2011.02.006
Sharma A, Houshyar R, Bhosale P, Choi JI, Gulati R, Lall C. Chemotherapy induced liver abnormalities: an imaging perspective. Clin Mol Hepatol. 2014;20(3):317. DOI: https://doi.org/10.3350/cmh.2014.20.3.317
Brancatelli G, Furlan A, Calandra A, Dioguardi Burgio M. Hepatic sinusoidal dilatation. Abdom Radiol. 2018;43(8):2011–22. DOI: https://doi.org/10.1007/s00261-018-1465-8
Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4(4):332–46. DOI: https://doi.org/10.1016/j.jceh.2014.10.002
El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd R, Gaur RL, Fernando A, et al. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci. 2009;466–73. DOI: https://doi.org/10.7150/ijbs.5.466
Nayak NC, Sathar SA, Mughal S, Duttagupta S, Mathur M, Chopra P. The nature and significance of liver cell vacuolation following hepatocellular injury ? An analysis based on observations on rats rendered tolerant to hepatotoxic damage. Virchows Arch. 1996;428(6). DOI: https://doi.org/10.1007/BF00202202
Krishna M. Patterns of necrosis in liver disease: Patterns of Necrosis in Liver Disease. Clin Liver Dis. 2017;10(2):53–6. DOI: https://doi.org/10.1002/cld.653
Box VGS. The intercalation of DNA double helices with doxorubicin and nagalomycin. J Mol Graph Model. 2007;26(1):14–9. DOI: https://doi.org/10.1016/j.jmgm.2006.09.005
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2023 Khulud Nurani, Anne Pulei, Beda Olabu, Jeremiah Munguti, Talha Chaudhry, Vincent Kipkorir

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- The Author retains copyright in the Work, where the term “Work” shall include all digital objects that may result in subsequent electronic publication or distribution.
- Upon acceptance of the Work, the author shall grant to the Publisher the right of first publication of the Work.
- The Author shall grant to the Publisher and its agents the nonexclusive perpetual right and license to publish, archive, and make accessible the Work in whole or in part in all forms of media now or hereafter known under a Creative Commons Attribution 4.0 International License or its equivalent, which, for the avoidance of doubt, allows others to copy, distribute, and transmit the Work under the following conditions:
- Attribution—other users must attribute the Work in the manner specified by the author as indicated on the journal Web site; with the understanding that the above condition can be waived with permission from the Author and that where the Work or any of its elements is in the public domain under applicable law, that status is in no way affected by the license.
- The Author is able to enter into separate, additional contractual arrangements for the nonexclusive distribution of the journal's published version of the Work (e.g., post it to an institutional repository or publish it in a book), as long as there is provided in the document an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post online a prepublication manuscript (but not the Publisher’s final formatted PDF version of the Work) in institutional repositories or on their Websites prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work. Any such posting made before acceptance and publication of the Work shall be updated upon publication to include a reference to the Publisher-assigned DOI (Digital Object Identifier) and a link to the online abstract for the final published Work in the Journal.
- Upon Publisher’s request, the Author agrees to furnish promptly to Publisher, at the Author’s own expense, written evidence of the permissions, licenses, and consents for use of third-party material included within the Work, except as determined by Publisher to be covered by the principles of Fair Use.
- The Author represents and warrants that:
- the Work is the Author’s original work;
- the Author has not transferred, and will not transfer, exclusive rights in the Work to any third party;
- the Work is not pending review or under consideration by another publisher;
- the Work has not previously been published;
- the Work contains no misrepresentation or infringement of the Work or property of other authors or third parties; and
- the Work contains no libel, invasion of privacy, or other unlawful matter.
- The Author agrees to indemnify and hold Publisher harmless from the Author’s breach of the representations and warranties contained in Paragraph 6 above, as well as any claim or proceeding relating to Publisher’s use and publication of any content contained in the Work, including third-party content.
Enforcement of copyright
The IJMS takes the protection of copyright very seriously.
If the IJMS discovers that you have used its copyright materials in contravention of the license above, the IJMS may bring legal proceedings against you seeking reparation and an injunction to stop you using those materials. You could also be ordered to pay legal costs.
If you become aware of any use of the IJMS' copyright materials that contravenes or may contravene the license above, please report this by email to contact@ijms.org
Infringing material
If you become aware of any material on the website that you believe infringes your or any other person's copyright, please report this by email to contact@ijms.org







