Diagnostic Performance of Western Blot for Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.5195/ijms.2025.3091Keywords:
Western Blot, Diagnosis, T. Gondii, Congenital ToxoplasmosisAbstract
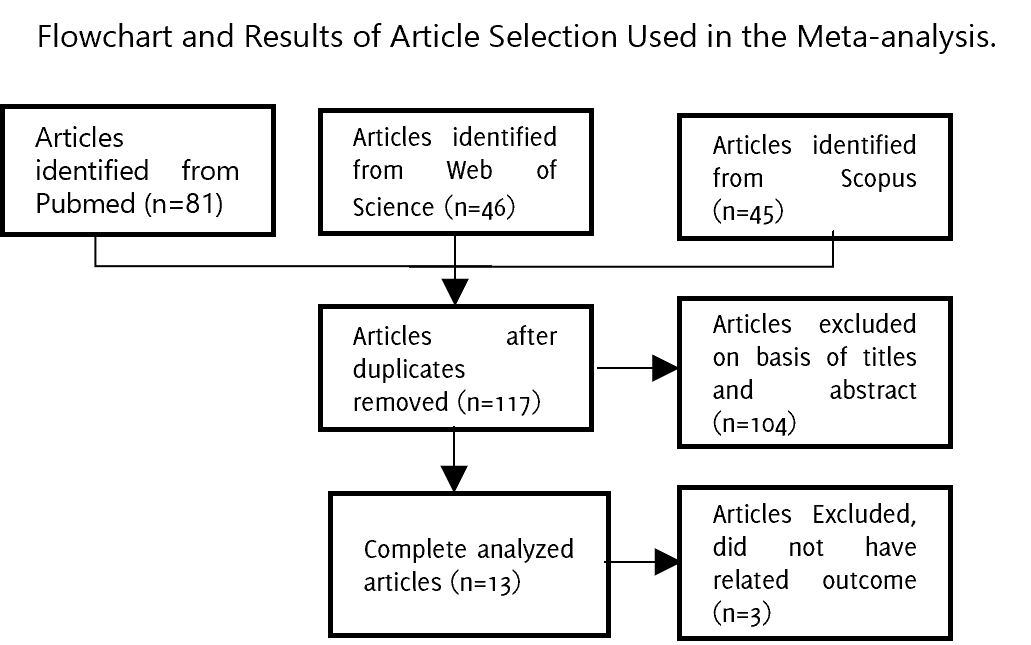
Background: Congenital toxoplasmosis results from infection with the parasite Toxoplasma gondii, which is transmitted from mother to child during pregnancy. Although Western blot is considered the most sensitive diagnostic tool for congenital toxoplasmosis, its diagnostic performance has not been subjected to meta-analysis. Methods: We conducted a systematic review and meta-analysis by performing literature searches across PubMed, Scopus, and Web of Science. The search strategy included the terms "western blot OR immunoblot" AND "congenital toxoplasmosis." The selected studies were required to meet specific inclusion criteria, which involved comparing the performance of the western blot test against the gold standard criteria for permanence of IgG after 10 months of age. These studies had to be case and control studies. The data obtained from the studies were then organized into an evidence synthesis table and the sensitivity, specificity, and Diagnostic Odds Ratio (DOR) index were calculated. This meta-analysis was performed in compliance with the recommendations of PRISMA guidelines. Results: After evaluating the selection criteria, we identified 44 articles; however, only 10 were selected for the meta-analysis. Western blot assay demonstrated a pooled sensitivity of 93.8% (95% CI: 79.2-98.4) and a pooled specificity of 96.6% (95% CI: 89.8-98.9) for the diagnosis of congenital toxoplasmosis. Six of the 10 studies had a DOR higher than 300, whereas the in-house method yielded a lower DOR of 1.2. Conclusions: This meta-analysis confirmed the utility of well-standardized western blot tests as a dependable diagnostic approach for congenital toxoplasmosis in terms of both sensitivity and specificity.
References
Mandelbrot, L., Gomez-Marin, J. Protozoan diseases: Toxoplasmosis. Reference Module in Biomedical Sciences, Elsevier. 2024, ISBN 9780128012383 https://doi.org/10.1016/B978-0-323-99967-0.00132-0.
Pinon JM, Dumon H, Chemla C, Franck J, Petersen E, Lebech M, et al. [Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J Clin Microbiol [Internet].2001:2267–71.https://doi.org/10.1128%2FJCM.39.6.2267-2271.2001.
Cortés J, Gómez-Marin J, Silva P, Arévalo L, Arévalo Rodríguez I, Isabel Alvarez M, et al. Clinical practice guideline. Integral Care Guidelines for the prevention, early detection and treatment of pregnancy, childbirth and puerperium complications: Section on toxoplasmosis in pregnancy. https://www.elsevier.es/es-revista-infectio-351-articulo-guia-atencion-integral-prevencion-deteccion-S0123939212700188.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med 18(3): e1003583. https://doi.org/10.1371/journal.pmed.1003583.
Capobiango JD, Monica TC, Ferreira FP, Mitsuka-Breganó R, Navarro IT, Garcia JL, et al. Evaluation of the western blotting method for the diagnosis of congenital toxoplasmosis. J Pediatr (Rio J) [Internet]. 2016;92(6):616–23. https://pubmed.ncbi.nlm.nih.gov/27504975/.
Machado AS, Andrade GM, Januário JN, Fernandes MD, Carneiro AC, Carneiro M, et al. IgG and IgM western blot assay for diagnosis of congenital toxoplasmosis. Mem Inst Oswaldo Cruz [Internet]. 2010;105(6):757-61. https://doi.org/10.1590/s0074-02762010000600005.
Gallego-Marín C, Henao AC, Gómez-Marín JE. Clinical validation of a western blot assay for congenital toxoplasmosis and newborn screening in a hospital in Armenia (Quindio) Colombia. J Trop Pediatr. [Internet]. 2005;52(2):107–12. https://pubmed.ncbi.nlm.nih.gov/16014760/.
L'Ollivier C, Wallon M, Faucher B, Piarroux R, Peyron F, Franck J. Comparison of mother and child antibodies that target high-molecular-mass Toxoplasma gondii antigens by immunoblotting improves neonatal diagnosis of congenital toxoplasmosis. Clin Vaccine Immunol [Internet]. 2012;19(8):1326-8. https://journals.asm.org/doi/10.1128/CVI.00060-12?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Magi B, Migliorini L. western blotting for the diagnosis of congenital toxoplasmosis. New Microbiol [Internet]. 2011;34(1):93–5. https://pubmed.ncbi.nlm.nih.gov/21344152/.
Franck J, Garin YJ, Dumon H. LDBio-Toxo II immunoglobulin G western blot confirmatory test for anti-toxoplasma antibody detection. J Clin Microbiol [Internet]. 2008;46(7):2334-8. http://dx.doi.org/10.1128/jcm.00182-08.
Tissot Dupont D, Fricker-Hidalgo H, Brenier-Pinchart MP, Bost-Bru C, Ambroise-Thomas P, Pelloux H. Usefulness of western blot in serological follow-up of newborns suspected of congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis [Internet]. 2003;22(2):122–5 http://dx.doi.org/10.1007/s10096-003-0887-5.
Rilling V, Dietz K, Krczal D, Knotek F, Enders G. Evaluation of a commercial IgG/IgM western blot assay for early postnatal diagnosis of congenital toxoplasmosis. Eur J Clin Microbiol Infect Dis [Internet]. 2003;22(3):174–80. http://dx.doi.org/10.1007/s10096-003-0906-6.
Stajner T, Bobic B, Klun I, Nikolic A, Srbljanovic J, Uzelac A, et al. Prenatal and early postnatal diagnosis of congenital toxoplasmosis in a setting with no systematic screening in pregnancy. Medicine (Baltimore) [Internet]. 2016;95(9):e2979.http://dx.doi.org/10.1097/md.0000000000002979.
Public Health England. Commercial and In-House Diagnostic Tests: Evaluations, Verifications and Validations. UK Standards for Microbiology Investigations. Q 1 Issue d. 2015. [Internet]. https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical-laboratories
Lemos Luengas Elkin Vladimir. Selección de la opción costo-efectiva para Colombia en la detección de toxoplasmosis congénita en el recién nacido. Infect. [Internet]. 2013 June [cited 2024 March 03] ; 17( 2 ): 51-52. Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-93922013000200001&lng=en.
Chicaíza-Becerra L, García-Molina M, Oviedo-Ariza S, Gómez-Marín JE, Gómez-Sánchez P. Costo efectividad de diferentes estrategias diagnósticas para detección de toxoplasmosis congénita en el recién nacido. Infectio 2013; 17 (2): 53-60. https://doi.org/10.1016/S0123-9392(13)70163-2.
Published
How to Cite
License
Copyright (c) 2025 Sebastián Serna Rivera, Maria Antonia Restrepo Duque, Jocelyn Arredondo Torres, Juan David Fandiño, Luis Felipe Mosquera, Jorge Enrique Gomez Marin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- The Author retains copyright in the Work, where the term “Work” shall include all digital objects that may result in subsequent electronic publication or distribution.
- Upon acceptance of the Work, the author shall grant to the Publisher the right of first publication of the Work.
- The Author shall grant to the Publisher and its agents the nonexclusive perpetual right and license to publish, archive, and make accessible the Work in whole or in part in all forms of media now or hereafter known under a Creative Commons Attribution 4.0 International License or its equivalent, which, for the avoidance of doubt, allows others to copy, distribute, and transmit the Work under the following conditions:
- Attribution—other users must attribute the Work in the manner specified by the author as indicated on the journal Web site; with the understanding that the above condition can be waived with permission from the Author and that where the Work or any of its elements is in the public domain under applicable law, that status is in no way affected by the license.
- The Author is able to enter into separate, additional contractual arrangements for the nonexclusive distribution of the journal's published version of the Work (e.g., post it to an institutional repository or publish it in a book), as long as there is provided in the document an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post online a prepublication manuscript (but not the Publisher’s final formatted PDF version of the Work) in institutional repositories or on their Websites prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work. Any such posting made before acceptance and publication of the Work shall be updated upon publication to include a reference to the Publisher-assigned DOI (Digital Object Identifier) and a link to the online abstract for the final published Work in the Journal.
- Upon Publisher’s request, the Author agrees to furnish promptly to Publisher, at the Author’s own expense, written evidence of the permissions, licenses, and consents for use of third-party material included within the Work, except as determined by Publisher to be covered by the principles of Fair Use.
- The Author represents and warrants that:
- the Work is the Author’s original work;
- the Author has not transferred, and will not transfer, exclusive rights in the Work to any third party;
- the Work is not pending review or under consideration by another publisher;
- the Work has not previously been published;
- the Work contains no misrepresentation or infringement of the Work or property of other authors or third parties; and
- the Work contains no libel, invasion of privacy, or other unlawful matter.
- The Author agrees to indemnify and hold Publisher harmless from the Author’s breach of the representations and warranties contained in Paragraph 6 above, as well as any claim or proceeding relating to Publisher’s use and publication of any content contained in the Work, including third-party content.
Enforcement of copyright
The IJMS takes the protection of copyright very seriously.
If the IJMS discovers that you have used its copyright materials in contravention of the license above, the IJMS may bring legal proceedings against you seeking reparation and an injunction to stop you using those materials. You could also be ordered to pay legal costs.
If you become aware of any use of the IJMS' copyright materials that contravenes or may contravene the license above, please report this by email to contact@ijms.org
Infringing material
If you become aware of any material on the website that you believe infringes your or any other person's copyright, please report this by email to contact@ijms.org







